Abstract
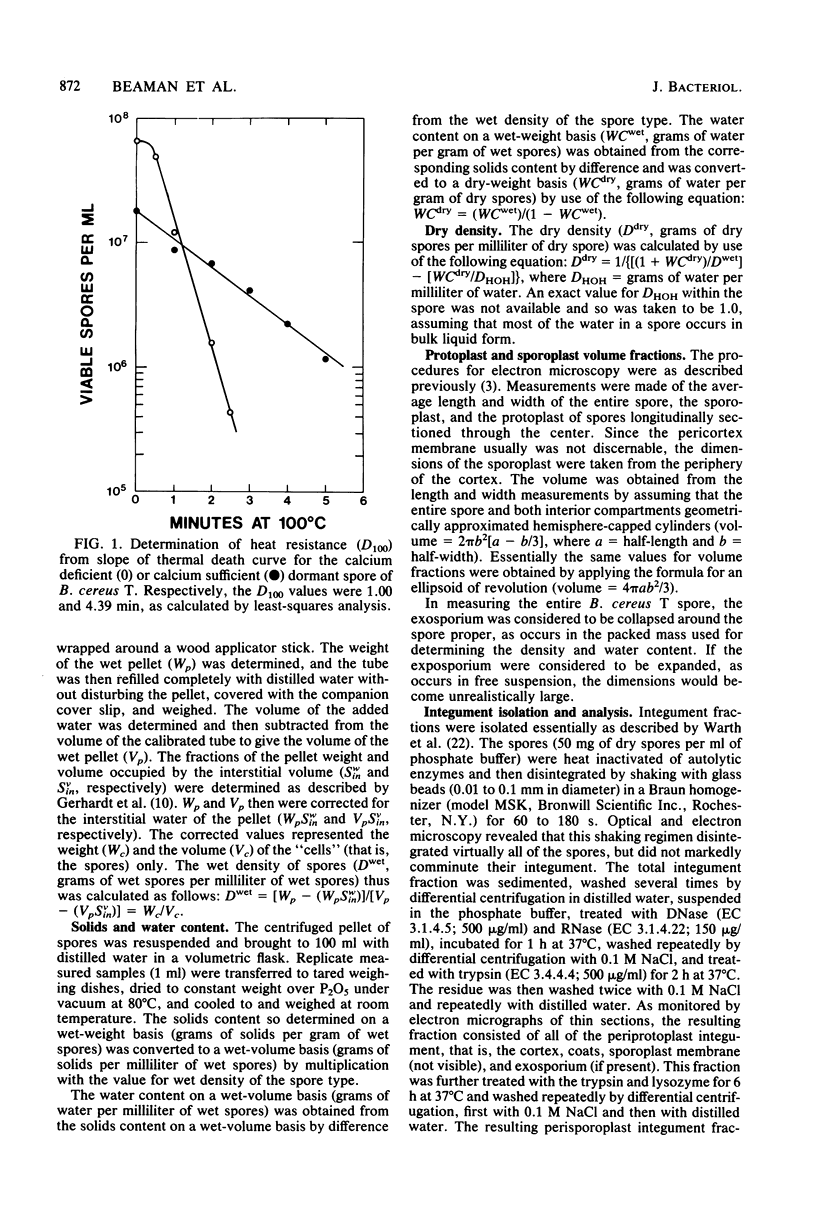
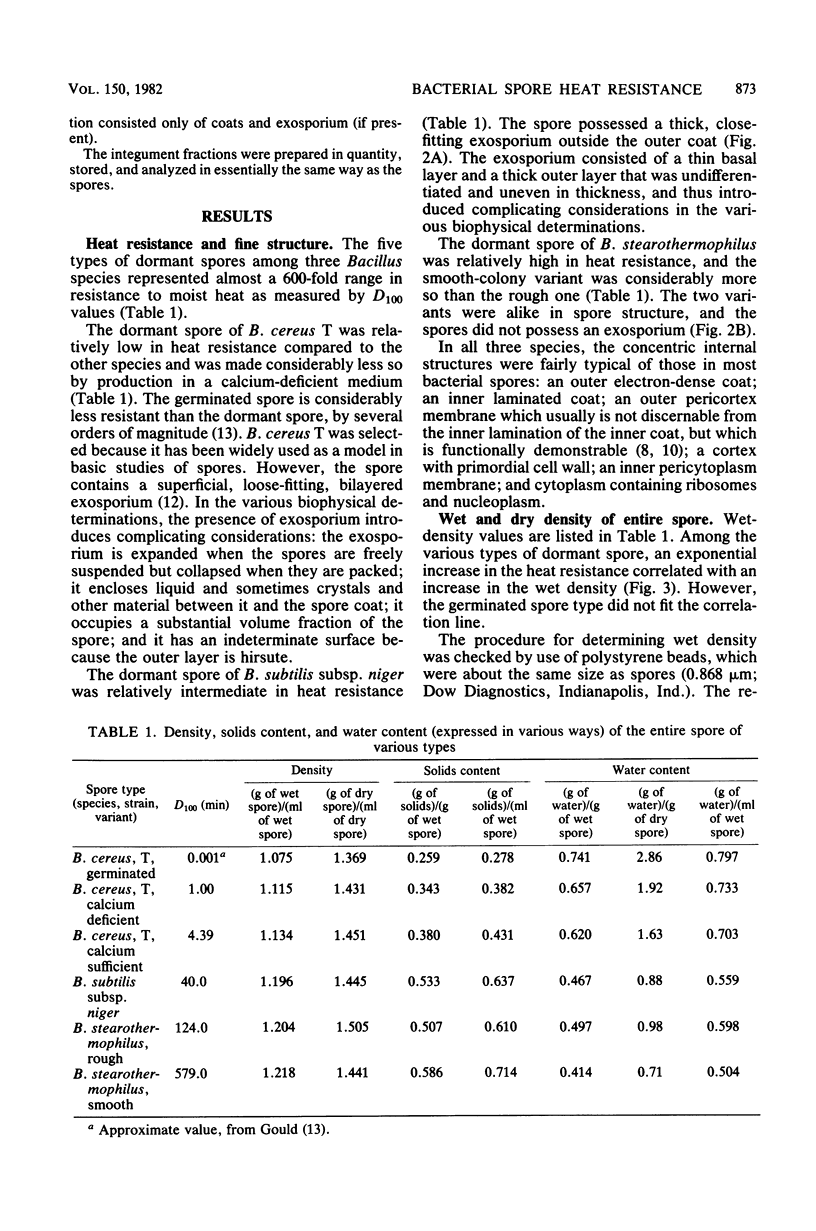
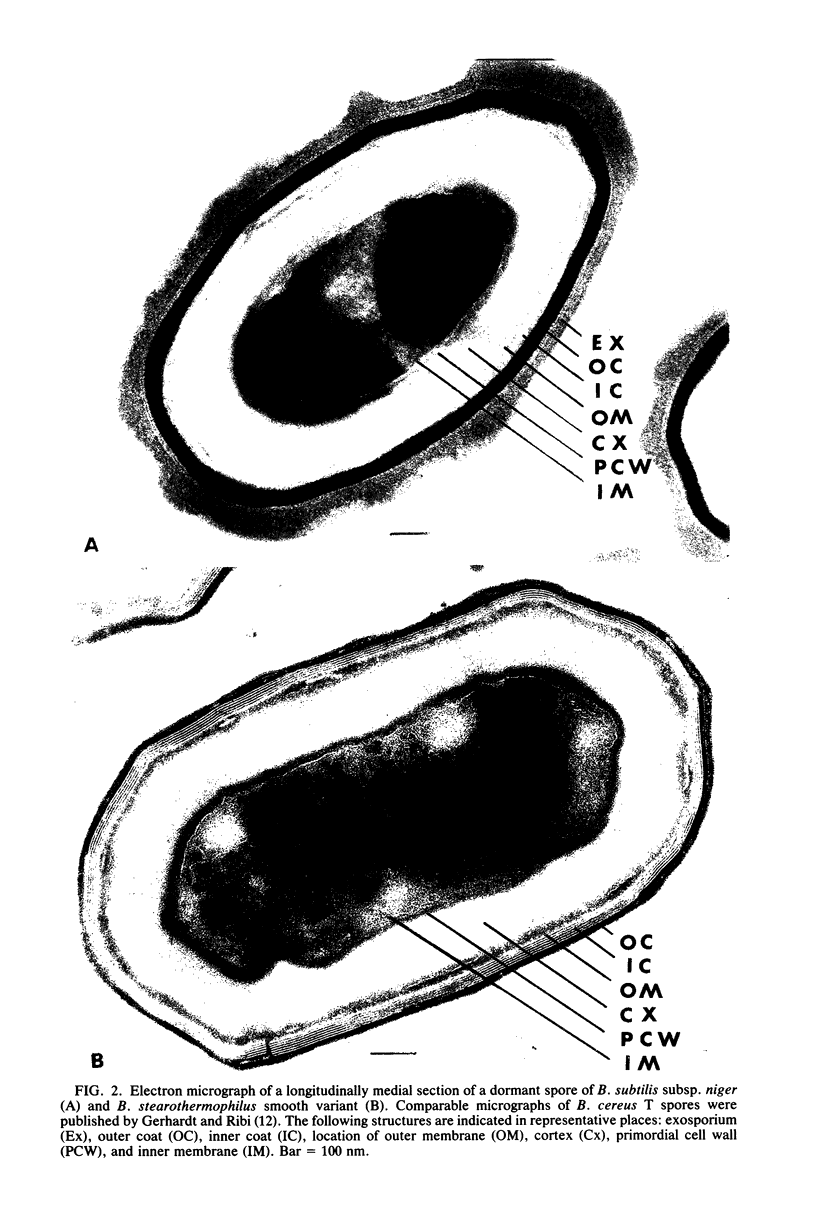
Five types of dormant Bacillus spores, between and within species, were selected representing a 600-fold range in moist-heat resistance determined as a D100 value. The wet and dry density and the solids and water content of the entire spore and isolated integument of each type were determined directly from gram masses of material, with correction for interstitial water. The ratio between the volume occupied by the protoplast (the structures bounded by the inner pericytoplasm membrane) and the volume occupied by the sporoplast (the structures bounded by the outer pericortex membrane) was calculated from measurements made on electron micrographs of medially thin-sectioned spores. Among the various spore types, an exponential increase in the heat resistance correlated directly with the wet density and inversely with the water content and with the protoplast/sporoplast volume ratio. Altogether with results supported a hypothesis that the extent of heat resistance is based in whole or in part on the extent of dehydration and diminution of the protoplast in the dormant spore, without implications about physiological mechanisms for attaining this state.
Full text
PDF

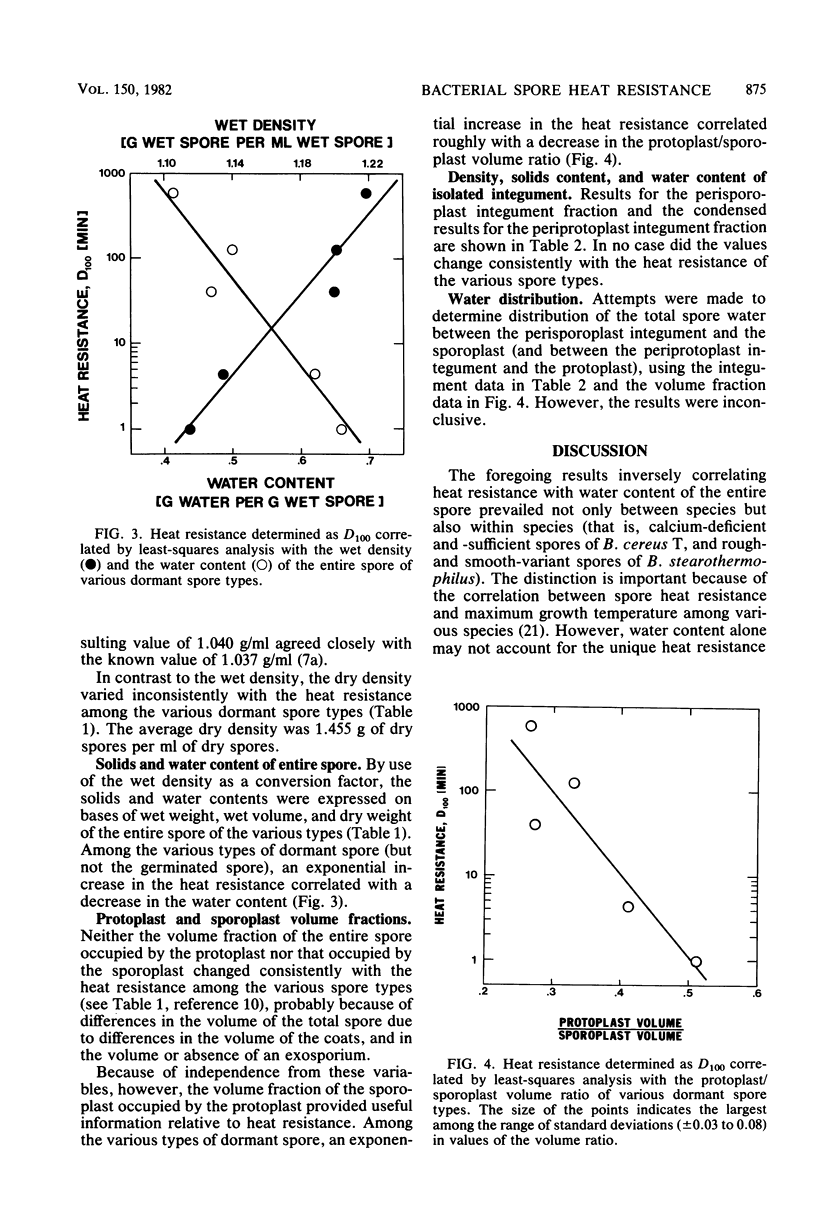
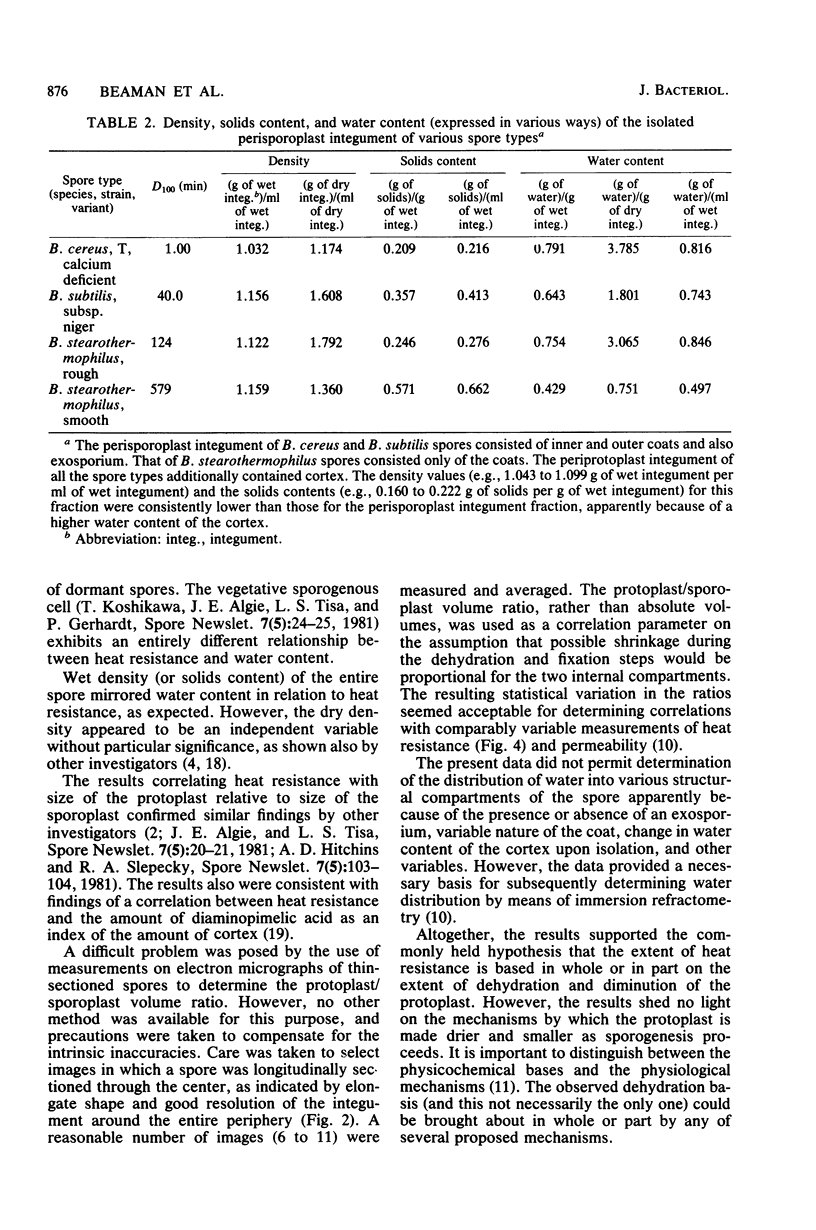

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. N., Lacy J. S. Permeability of the cell envelope and osmotic behavior in Saccharomyces cerevisiae. J Bacteriol. 1977 Aug;131(2):564–571. doi: 10.1128/jb.131.2.564-571.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERLIN E., CURRAN H. R., PALLANSCH M. J. PHYSICAL SURFACE FEATURES AND CHEMICAL DENSITY OF DRY BACTERIAL SPORES. J Bacteriol. 1963 Nov;86:1030–1036. doi: 10.1128/jb.86.5.1030-1036.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. I. Characterization of glucose uptake. J Bacteriol. 1961 Nov;82:743–749. doi: 10.1128/jb.82.5.743-749.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. III. Permeation relative to germination. J Bacteriol. 1962 Feb;83:301–308. doi: 10.1128/jb.83.2.301-308.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. IV. Water content, uptake, and distribution. J Bacteriol. 1962 May;83:960–967. doi: 10.1128/jb.83.5.960-967.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman T. C., Pankratz H. S., Gerhardt P. Ultrastructure of the exosporium and underlying inclusions in spores of Bacillus megaterium strains. J Bacteriol. 1972 Mar;109(3):1198–1209. doi: 10.1128/jb.109.3.1198-1209.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury J. H., Foster J. R., Hammer B., Lindsay J., Murrell W. G. The source of the heat resistance of bacterial spores. Study of water in spores by NMR. Biochim Biophys Acta. 1981 Dec 4;678(2):157–164. doi: 10.1016/0304-4165(81)90201-4. [DOI] [PubMed] [Google Scholar]
- Crafts-Lighty A., Ellar D. J. The structure and function of the spore outer membrane in dormant and germinating spores of Bacillus megaterium. J Appl Bacteriol. 1980 Feb;48(1):135–145. doi: 10.1111/j.1365-2672.1980.tb05215.x. [DOI] [PubMed] [Google Scholar]
- FIELDS M. L. Effect of heat on spores of rough and smooth variants of Bacillus stearothermophilus. Appl Microbiol. 1963 Mar;11:100–104. doi: 10.1128/am.11.2.100-104.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., RIBI E. ULTRASTRUCTURE OF THE EXOSPORIUM ENVELOPING SPORES OF BACILLUS CEREUS. J Bacteriol. 1964 Dec;88:1774–1789. doi: 10.1128/jb.88.6.1774-1789.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt P., Beaman T. C., Corner T. R., Greenamyre J. T., Tisa L. S. Photometric immersion refractometry of bacterial spores. J Bacteriol. 1982 May;150(2):643–648. doi: 10.1128/jb.150.2.643-648.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G. W. Recent advances in the understanding of resistance and dormancy in bacterial spores. J Appl Bacteriol. 1977 Jun;42(3):297–309. doi: 10.1111/j.1365-2672.1977.tb00697.x. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO T., BLACK S. H., GERHARDT P. Development of fine structure, thermostability, and dipicolinate during sporogenesis in a bacillus. Can J Microbiol. 1960 Apr;6:203–212. doi: 10.1139/m60-022. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Murrell W. J. Symposium on bacterial spores: IX. Biophysical analysis of the spore. J Appl Bacteriol. 1970 Mar;33(1):103–129. doi: 10.1111/j.1365-2672.1970.tb05237.x. [DOI] [PubMed] [Google Scholar]
- RODE L. J., LEWIS C. W., Jr, FOSTER J. W. Electron microscopy of spores of Bacillus megaterium with special reference to the effects of fixation and thin sectioning. J Cell Biol. 1962 Jun;13:423–435. doi: 10.1083/jcb.13.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. Location and composition of spore mucopeptide in Bacillus species. J Cell Biol. 1963 Mar;16:593–609. doi: 10.1083/jcb.16.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D. Relationship between the heat resistance of spores and the optimum and maximum growth temperatures of Bacillus species. J Bacteriol. 1978 Jun;134(3):699–705. doi: 10.1128/jb.134.3.699-705.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]