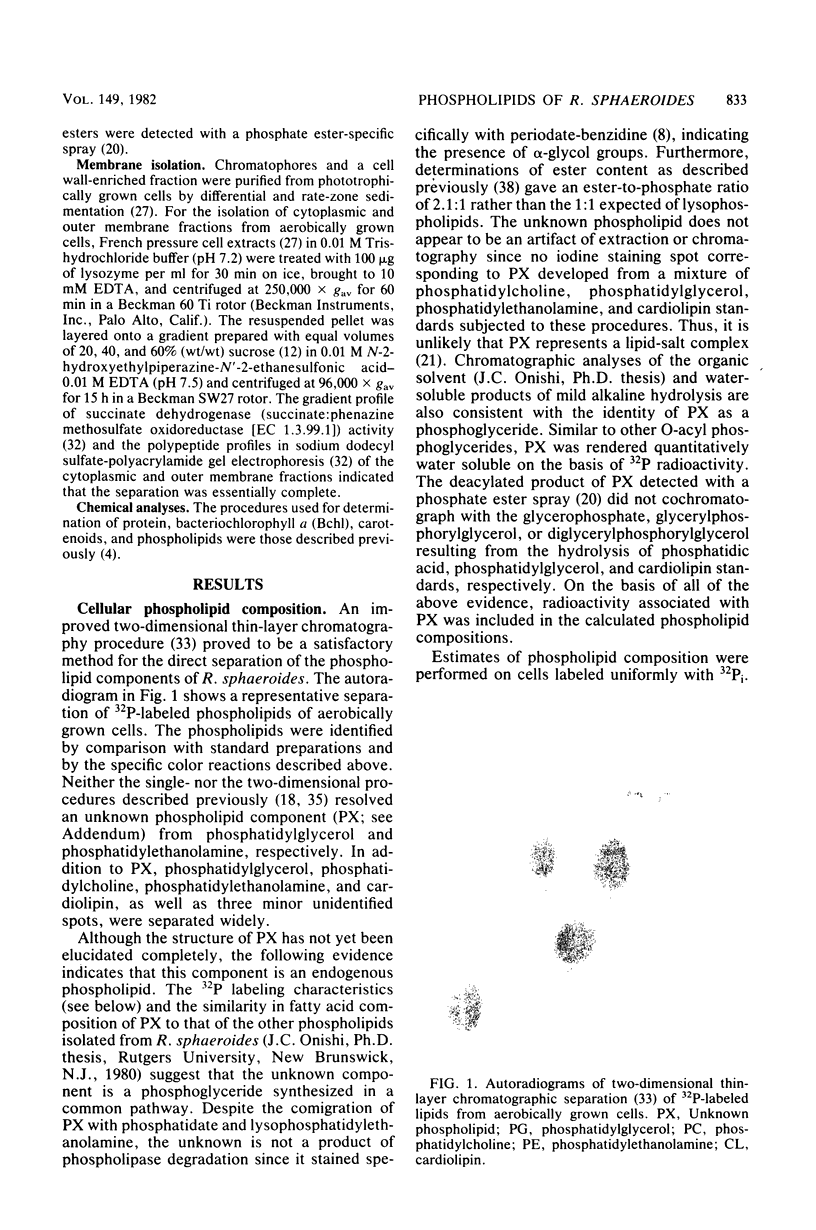
Abstract
The effects of growth conditions on phospholipid composition in Rhodopseudomonas sphaeroides have been reexamined. The levels of phosphatidylethanolamine (27 to 28%), phosphatidylglycerol (23 to 24%), and phosphatidylcholine (11 to 18%) were very similar in cells grown aerobically or phototrophically at a high light intensity, consistent with findings for another member of Rhodospirillaceae. In addition, an unknown phospholipid species was detected which comprised 20 to 30% of the total phospholipid in these cells. In cells growing phototrophically at low-intensity illumination, the level of phosphatidylethanolamine increased by about 1.6-fold and that of the unknown phospholipid markedly decreased. Although the synthesis of photosynthetic pigments, light-harvesting protein, and intracytoplasmic photosynthetic membranes also increased markedly, the ratios of individual phospholipid species were essentially identical in photosynthetic membrane and cell wall fractions purified from these cells. Since a significant exchange of lipids apparently did not occur during the isolation of these fractions, it was suggested that the changes in cellular phospholipid accumulation were not due to a unique composition within the photosynthetic membrane. Instead, these phosphoglyceride changes were found to be related to overall phospholipid metabolism and could be accounted for principally by differences in biosynthetic rates. These results, together with studies in nutrient-restricted aerobic cells, suggested that the mechanism by which phospholipid levels are regulated may be related to radiant energy flux rather than cellular energy limitation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell G. B., Sistrom W. R., Griffith O. H. Lipid-protein associations in chromatophores from the photosynthetic bacterium Rhodopseudomonas sphaeroides. Biochemistry. 1978 Sep 5;17(18):3768–3773. doi: 10.1021/bi00611a015. [DOI] [PubMed] [Google Scholar]
- Broglie R. M., Hunter C. N., Delepelaire P., Niederman R. A., Chua N. H., Clayton R. K. Isolation and characterization of the pigment-protein complexes of Rhodopseudomonas sphaeroides by lithium dodecyl sulfate/polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):87–91. doi: 10.1073/pnas.77.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie R. M., Niederman R. A. Membranes of Rhodopseudomonas sphaeroides: effect of cerulenin on assembly of chromatophore membrane. J Bacteriol. 1979 Jun;138(3):788–798. doi: 10.1128/jb.138.3.788-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. E., Eiserling F. A., Lascelles J. Bacteriochlorophyll Synthesis and the Ultrastructure of Wild Type and Mutant Strains of Rhodopseudomonas spheroides. Plant Physiol. 1972 Dec;50(6):743–746. doi: 10.1104/pp.50.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain B. D., Deal C. D., Fraley R. T., Kaplan S. In vivo intermembrane transfer of phospholipids in the photosynthetic bacterium Rhodopseudomonas sphaeroides. J Bacteriol. 1981 Mar;145(3):1154–1166. doi: 10.1128/jb.145.3.1154-1166.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain B. K., Webster R. E. Effect of membrane-associated f1 bacteriophage coat protein upon the activity of Escherichia coli phosphatidylserine synthetase. J Bacteriol. 1978 Sep;135(3):883–887. doi: 10.1128/jb.135.3.883-887.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K., Haselkorn R. Protein components of bacterial photosynthetic membranes. J Mol Biol. 1972 Jul 14;68(1):97–105. doi: 10.1016/0022-2836(72)90265-3. [DOI] [PubMed] [Google Scholar]
- Collins M. L., Mallon D. E., Niederman R. A. Assessment of Rhodopseudomonas sphaeroides chromatophore membrane asymmetry through bilateral antiserum adsorption studies. J Bacteriol. 1980 Jul;143(1):221–230. doi: 10.1128/jb.143.1.221-230.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. L., Niederman R. A. Membranes of Rhodospirillum rubrum: isolation and physicochemical properties of membranes from aerobically grown cells. J Bacteriol. 1976 Jun;126(3):1316–1325. doi: 10.1128/jb.126.3.1316-1325.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Ding D. H., Kaplan S. Separation of inner and outer membranes of Rhodopseudomonas spheroides. Prep Biochem. 1976;6(1):61–79. doi: 10.1080/00327487608061599. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Kaplan S. Isolation and characterization of a bacteriochlorophyll-containing protein from Rhodopseudomonas spheroides. J Biol Chem. 1972 May 10;247(9):2732–2737. [PubMed] [Google Scholar]
- Fraley R. T., Yen G. S., Lueking D. R., Kaplan S. The physical state of the intracytoplasmic membrane of Rhodopseudomonas sphaeroides and its relationship to the cell division cycle. J Biol Chem. 1979 Mar 25;254(6):1987–1991. [PubMed] [Google Scholar]
- Francis G. A., Richards W. R. Localization of photosynthetic membrane components in Rhodopseudomonas sphaeroides by a radioactive labeling procedure. Biochemistry. 1980 Oct 28;19(22):5104–5111. doi: 10.1021/bi00563a026. [DOI] [PubMed] [Google Scholar]
- Golecki J. R., Schumacher A., Drews G. The differentiation of the photosynthetic apparatus and the intracytoplasmic membrane in cells of Rhodopseudomonas capsulata upon variation of light intensity. Eur J Cell Biol. 1980 Dec;23(1):1–5. [PubMed] [Google Scholar]
- Gorchein A. The separation and identification of the lipids of Rhodopseudomonas spheroides. Proc R Soc Lond B Biol Sci. 1968 Jul 2;170(1020):279–297. doi: 10.1098/rspb.1968.0039. [DOI] [PubMed] [Google Scholar]
- HANDS A. R., BARTLEY W. The fatty acids of Rhodopseudomonas particles. Biochem J. 1962 Aug;84:238–239. doi: 10.1042/bj0840238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Kanemasa Y., Akamatsu Y., Nojima S. Composition and turnover of the phospholipids in Escherichia coli. Biochim Biophys Acta. 1967 Oct 2;144(2):382–390. [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J., SZILAGYI J. F. PHOSPHOLIPID SYNTHESIS BY RHODOPSEUDOMONAS SPHEROIDES IN RELATION TO THE FORMATION OF PHOTOSYNTHETIC PIGMENTS. J Gen Microbiol. 1965 Jan;38:55–64. doi: 10.1099/00221287-38-1-55. [DOI] [PubMed] [Google Scholar]
- Lommen M. A., Takemoto J. Comparison, by freeze-fracture electron microscopy, of chromatophores, spheroplast-derived membrane vesicles, and whole cells of Rhodopseudomonas sphaeroides. J Bacteriol. 1978 Nov;136(2):730–741. doi: 10.1128/jb.136.2.730-741.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Oelze J., Golecki J. R., Kleinig H., Weckesser J. Characterization of two cell-envelope fractions from chemotrophically grown Rhodospirillum rubrum. Antonie Van Leeuwenhoek. 1975;41(3):273–286. doi: 10.1007/BF02565063. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Steiner L. A., Feher G. Characterization of reaction centers from photosynthetic bacteria. I. Subunit structure of the protein mediating the primary photochemistry in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1394–1403. doi: 10.1021/bi00704a013. [DOI] [PubMed] [Google Scholar]
- Ono Y., White D. C. Cardiolipin-specific phospholipase D activity in Haemophilus parainfluenzae. J Bacteriol. 1970 Jul;103(1):111–115. doi: 10.1128/jb.103.1.111-115.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks L. C., Niederman R. A. Membranes of Rhodopseudomonas sphaeroides. V. Identification of bacteriochlorophyll alpha-depleted cytoplasmic membrane in phototrophically grown cells. Biochim Biophys Acta. 1978 Jul 20;511(1):70–82. doi: 10.1016/0005-2736(78)90065-2. [DOI] [PubMed] [Google Scholar]
- Poorthuis B. J., Yazaki P. J., Hostetler K. Y. An improved two dimensional thin-layer chromatography system for the separation of phosphatidylglycerol and its derivatives. J Lipid Res. 1976 Jul;17(4):433–437. [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- Russell N. J., Harwood J. L. Changes in the acyl lipid composition of photosynthetic bacteria grown under photosynthetic and non-photosynthetic conditions. Biochem J. 1979 Aug 1;181(2):339–345. doi: 10.1042/bj1810339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN I., SHAPIRO B. A rapid and simple method for the determination of esterified fatty acids and for total fatty acids in blood. J Clin Pathol. 1953 May;6(2):158–160. doi: 10.1136/jcp.6.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Relation of turnover of membrane phospholipids to synthesis of membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4250–4255. [PubMed] [Google Scholar]
- Steiner S., Sejka G. A., Conti S. F., Gest H., Lester R. L. Modification of membrane composition in growing photosynthetic bacteria. Biochim Biophys Acta. 1970 Jun 2;203(3):571–574. doi: 10.1016/0005-2736(70)90194-x. [DOI] [PubMed] [Google Scholar]
- Takemoto J., Huang Kao M. Y. Effects of incident light levels on photosynthetic membrane polypeptide composition and assembly in Rhodopseudomonas sphaeroides. J Bacteriol. 1977 Feb;129(2):1102–1109. doi: 10.1128/jb.129.2.1102-1109.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto J., Lascelles J. Coupling between bacteriochlorophyll and membrane protein synthesis in Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):799–803. doi: 10.1073/pnas.70.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. A., Lennarz W. J., Schnaitman C. A. Distribution of lipids in the wall and cytoplasmic membrane subfractions of the cell envelope of Escherichia coli. J Bacteriol. 1972 Feb;109(2):686–690. doi: 10.1128/jb.109.2.686-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]