Abstract
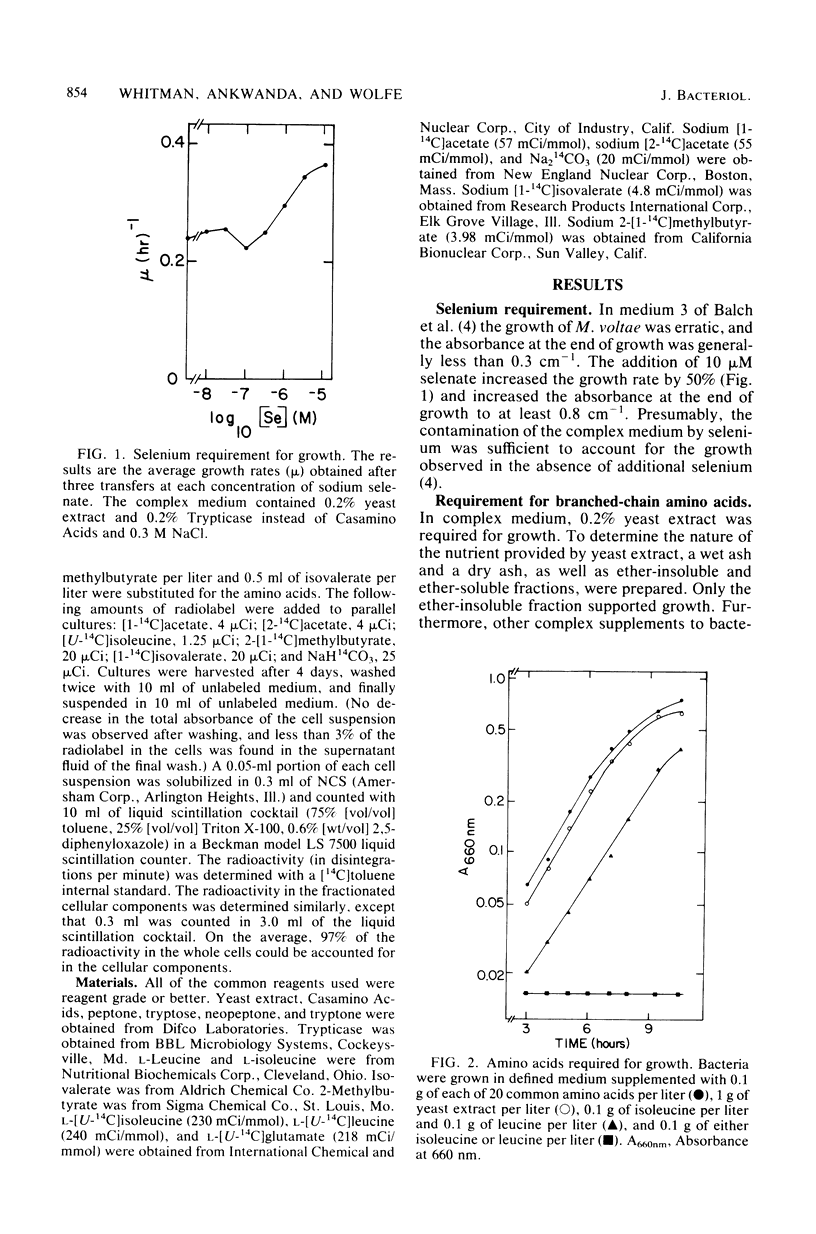
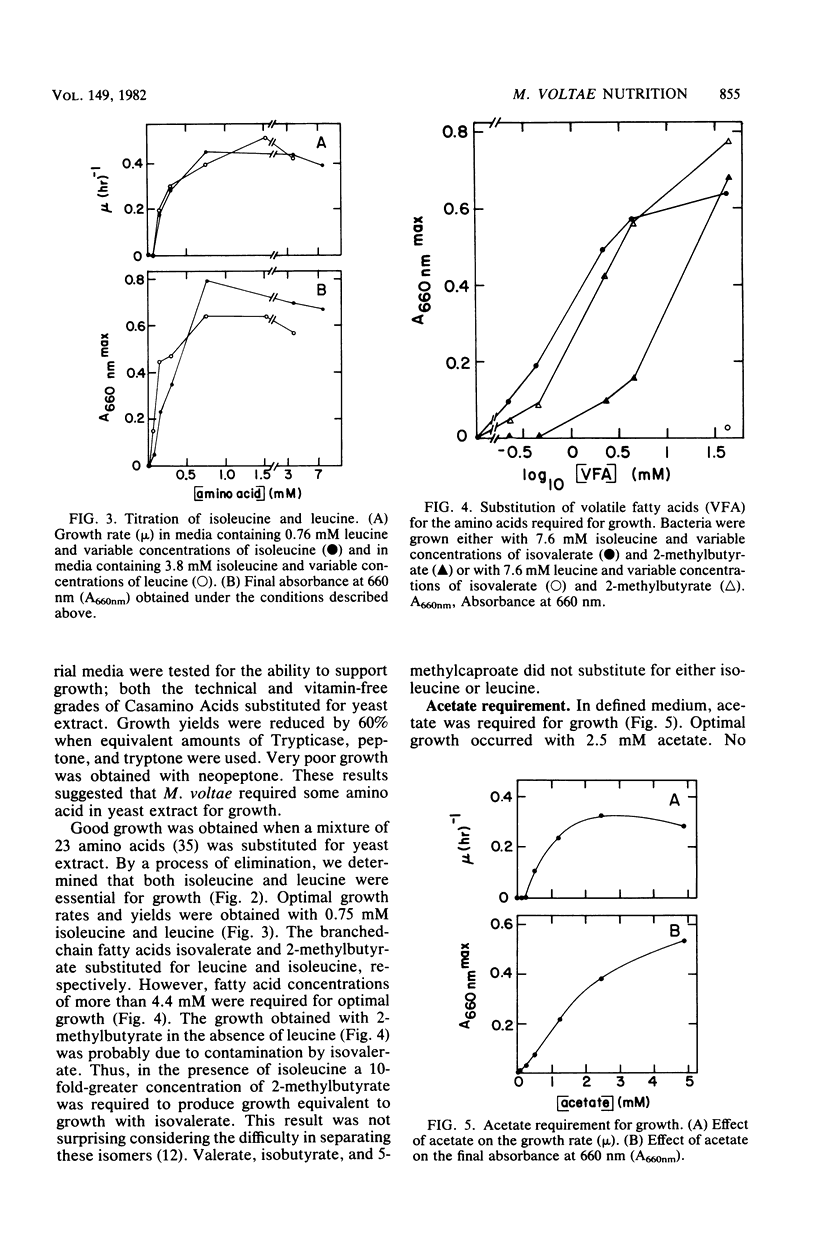
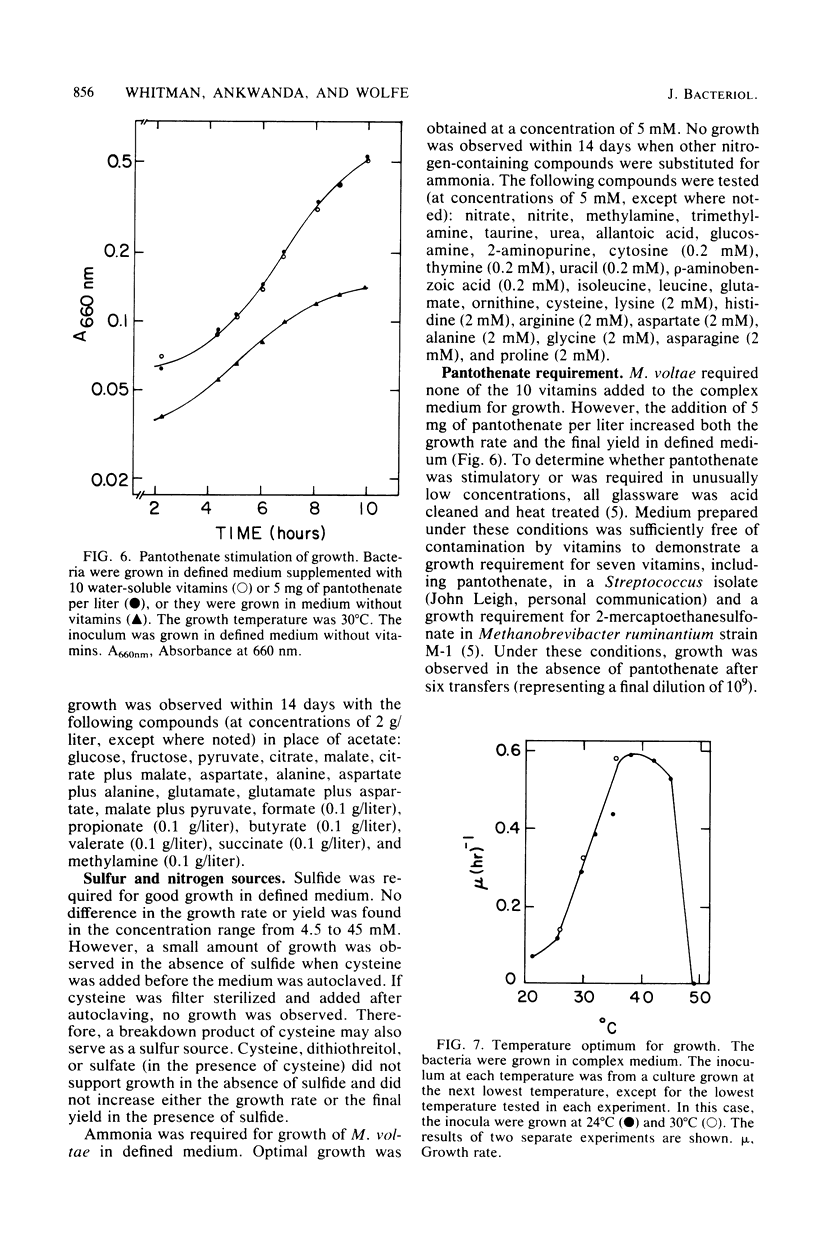
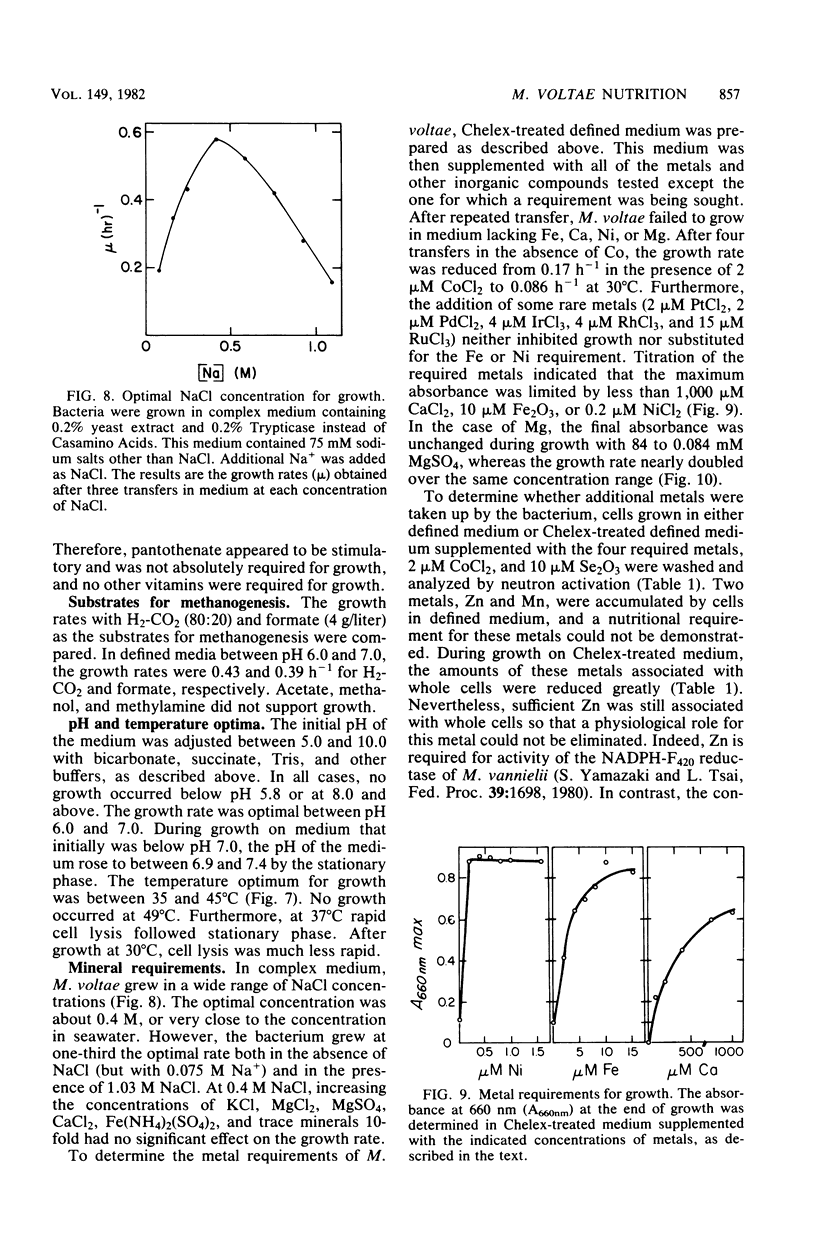
Methanococcus voltae is a heterotrophic, H2-oxidizing methanogenic bacterium. In complex medium, this bacterium has a doubling time of 1.2 h at its temperature optimum of 38 degrees C. In defined medium, optimal growth is obtained with 0.75 mM isoleucine, 0.75 mM leucine, 2.5 mM acetate, 5 mM NH4Cl, 84 mM MgSO4, 0.4 M NaCl, 1 mM CaCl2, 10 microM Fe2O3, and 0.2 microM NiCl2. In addition, pantothenate, sodium selenate, and cobalt stimulate growth. Optimal growth is obtained between pH 6.0 and 7.0 with either H2 or formate as the electron donor. The volatile fatty acids 2-methylbutyrate and isovalerate can substitute for isoleucine and leucine, respectively. Cellular carbon is derived from acetate (31%), isoleucine (22%), leucine (25%), and carbon dioxide (23%). The amino acids and fatty acids are incorporated almost exclusively into protein. A comparison of the incorporation of U-14C-amino acids and 1-14C-fatty acids indicated that the fatty acids are degraded during incorporation into cell protein. The distribution of carbon from the amino acids suggests that acetyl coenzyme A is not a major intermediate in the degradation of these compounds. Thus, M. voltae may convert isoleucine and leucine to other amino acids by a unique mechanism. The lipid carbon is derived largely from acetate. Thus, the isoprenoid lipids are synthesized de novo from acetate rather than by degradation of leucine. The carbon in the nucleic acids is derived from carbon dioxide (45%), the C-1 of acetate (25%), the C-2 of acetate (22%), and isoleucine and leucine (7%). This labeling pattern is consistent with known biochemical pathways.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P., DOETSCH R. N. Studies on the metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. I. Incorporation of isovalerate into leucine. J Bacteriol. 1962 Mar;83:523–532. doi: 10.1128/jb.83.3.523-532.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON M. J., BRYANT M. P., KATZ I., KEENEY M. Metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. II. Biosynthesis of higher branched-chain fatty acids and aldehydes. J Bacteriol. 1962 May;83:1084–1093. doi: 10.1128/jb.83.5.1084-1093.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., Peel J. L. The biosynthesis of valine from isobutyrate by peptostreptococcus elsdenii and Bacteroides ruminicola. Biochem J. 1971 Feb;121(3):431–437. doi: 10.1042/bj1210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best A. N. Composition and Characterization of tRNA from Methanococcus vannielii. J Bacteriol. 1978 Jan;133(1):240–250. doi: 10.1128/jb.133.1.240-250.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuil C., Patel G. B. Composition of Methanospirillum hungatii GP1 during growth on different media. Can J Microbiol. 1980 May;26(5):577–582. doi: 10.1139/m80-102. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Dehority B. A., Scott H. W., Kowaluk P. Volatile fatty acid requirements of cellulolytic rumen bacteria. J Bacteriol. 1967 Sep;94(3):537–543. doi: 10.1128/jb.94.3.537-543.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G., Klee B., Thauer R. K. Nickel, a component of factor F430 from Methanobacterium thermoautotrophicum. Arch Microbiol. 1980 Jan;124(1):103–106. doi: 10.1007/BF00407036. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Royle L. G., Murray L. Cationic composition of 22 species of bacteria grown in seawater medium. Appl Environ Microbiol. 1979 Nov;38(5):800–805. doi: 10.1128/aem.38.5.800-805.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Bowers B., Stadtman T. C. Methanococcus vannielii: ultrastructure and sensitivity to detergents and antibiotics. J Bacteriol. 1977 Jun;130(3):1357–1363. doi: 10.1128/jb.130.3.1357-1363.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Stadtman T. C. Methanococcus vannielii: culture and effects of selenium and tungsten on growth. J Bacteriol. 1977 Jun;130(3):1404–1406. doi: 10.1128/jb.130.3.1404-1406.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Stadtman T. C. Reconstitution of a formate-NADP+ oxidoreductase from formate dehydrogenase and a 5-deazaflavin-linked NADP+ reductase isolated from Methanococcus vannielii. J Biol Chem. 1980 Feb 10;255(3):1049–1053. [PubMed] [Google Scholar]
- Jones J. B., Stadtman T. C. Selenium-dependent and selenium-independent formate dehydrogenases of Methanococcus vannielii. Separation of the two forms and characterization of the purified selenium-independent form. J Biol Chem. 1981 Jan 25;256(2):656–663. [PubMed] [Google Scholar]
- Mah R. A., Ward D. M., Baresi L., Glass T. L. Biogenesis of methane. Annu Rev Microbiol. 1977;31:309–341. doi: 10.1146/annurev.mi.31.100177.001521. [DOI] [PubMed] [Google Scholar]
- Patel G. B., Khan A. W., Roth L. A. Optimum levels of sulphate and iron for the cultivation of pure cultures of methanogens in synthetic media. J Appl Bacteriol. 1978 Dec;45(3):347–356. doi: 10.1111/j.1365-2672.1978.tb04235.x. [DOI] [PubMed] [Google Scholar]
- Patel G. B., Roth L. A., van den Berg L., Clark D. S. Characterization of a strain of Methanospirillum hungatti. Can J Microbiol. 1976 Sep;22(9):1404–1410. doi: 10.1139/m76-208. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Allison M. J. Isoleucine biosynthesis from 2-methylbutyric acid by anaerobic bacteria from the rumen. J Bacteriol. 1969 Mar;97(3):1220–1226. doi: 10.1128/jb.97.3.1220-1226.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN T. C., BARKER H. A. Studies on the methane fermentation. X. A new formate-decomposing bacterium, Methanococcus vannielii. J Bacteriol. 1951 Sep;62(3):269–280. doi: 10.1128/jb.62.3.269-280.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönheit P., Moll J., Thauer R. K. Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch Microbiol. 1979 Oct;123(1):105–107. doi: 10.1007/BF00403508. [DOI] [PubMed] [Google Scholar]
- Settle D. M., Patterson C. C. Lead in albacore: guide to lead pollution in Americans. Science. 1980 Mar 14;207(4436):1167–1176. doi: 10.1126/science.6986654. [DOI] [PubMed] [Google Scholar]
- TAKACS F. P., MATULA T. I., MACLEOD R. A. NUTRITION AND METABOLISM OF MARINE BACTERIA. XIII. INTRACELLULAR CONCENTRATIONS OF SODIUM AND POTASSIUM IONS IN A MARINE PSEUDOMONAD. J Bacteriol. 1964 Mar;87:510–518. doi: 10.1128/jb.87.3.510-518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. T., Pirt S. J. Nutrition and factors limiting the growth of a methanogenic bacterium (Methanobacterium thermoautotrophicum). Arch Microbiol. 1977 May 13;113(1-2):17–22. doi: 10.1007/BF00428574. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Langworthy T. A. Diphytanyl and dibiphytanyl glycerol ether lipids of methanogenic archaebacteria. Science. 1979 Jan 5;203(4375):51–53. doi: 10.1126/science.758677. [DOI] [PubMed] [Google Scholar]
- WEGNER G. H., FOSTER E. M. Incorporation of isobutyrate and valerate into cellular plasmalogen by Bacteroides succinogenes. J Bacteriol. 1963 Jan;85:53–61. doi: 10.1128/jb.85.1.53-61.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. M., Mah R. A., Kaplan I. R. Methanogenesis from acetate: a nonmethanogenic bacterium from an anaerobic acetate enrichment. Appl Environ Microbiol. 1978 Jun;35(6):1185–1192. doi: 10.1128/aem.35.6.1185-1192.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Presence of nickel in factor F430 from Methanobacterium bryantii. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1196–1201. doi: 10.1016/0006-291x(80)90413-1. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


