Abstract
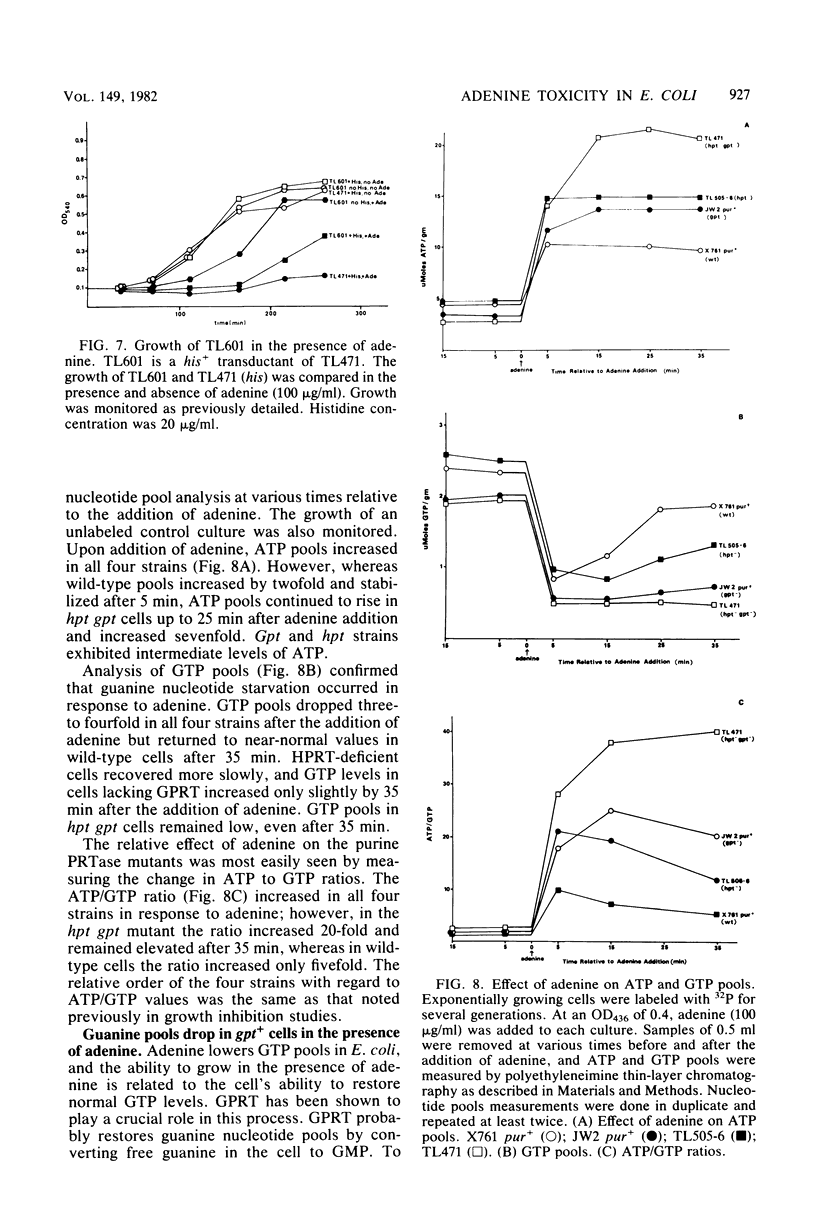
The mechanism of adenine toxicity in an hpt gpt strain of Escherichia coli that is extremely sensitive to adenine inhibition was investigated. Adenine-resistant derivatives had secondary mutations in adeninephosphoribosyltransferase or the purR repressor. Growth studies with various purine salvage pathway mutants and the ability of guanosine to prevent adenine toxicity indicated that adenine exerts its toxic effects by depleting guanine nucleotide pools. In the presence of adenine, ATP pools increased twofold in wild-type cells and stabilized after 5 min. In contrast, ATP pools continued to rise in hpt gpt cells up to 25 min and increased sevenfold after adenine addition. hpt gpt cells were shown to have higher levels of adeninephosphoribosyltransferase than did the wild-type cells. In response to adenine addition, GTP pools dropped three- to fourfold in all strains tested. Although GTP levels returned to near normal values in wild-type cells after 35 min, no restoration of GTP pools was observed in the hpt gpt strain during this period. Measurements of guanine pools before and after the addition of adenine indicated that guaninephosphoribosyltransferase plays an important role in maintaining GTP pools by converting the free guanine to GMP during guanine nucleotide depletion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKE M. S., MAGASANIK B. The metabolism of purines in Aerobacter aerogenes: a study of purineless mutants. J Bacteriol. 1954 Dec;68(6):727–733. doi: 10.1128/jb.68.6.727-733.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Purine phosphoribosyltransferases of Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):1010–1013. doi: 10.1128/jb.112.2.1010-1013.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal F. R., Gots J. S. Requirement of GTP for pteridine synthesis in Salmonella typhimurium and its inhibition by AMP. Biochem Biophys Res Commun. 1965 Aug 16;20(4):509–514. doi: 10.1016/0006-291x(65)90609-1. [DOI] [PubMed] [Google Scholar]
- Dalal F. R., Gots R. E., Gots J. S. Mechanism of adenine inhibition in adenine-sensitive mutants of Salmonella typhimurium. J Bacteriol. 1966 Feb;91(2):507–513. doi: 10.1128/jb.91.2.507-513.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Shumas S. R. Genetic separation of hypoxanthine and guanine-xanthine phosphoribosyltransferase activities by deletion mutations in Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):910–916. doi: 10.1128/jb.112.2.910-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey H. V., Taylor M. W. Purification of adenine phosphoribosyltransferase by affinity chromatography. Prep Biochem. 1978;8(6):453–462. doi: 10.1080/00327487808061662. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. I. Purification of adenine phosphoribosyltransferase from Escherichia coli K12 and control of activity by nucleotides. J Biol Chem. 1971 Sep 10;246(17):5294–5303. [PubMed] [Google Scholar]
- Holden J. A., Harriman P. D., Wall J. D. Escherichia coli mutants deficient in guanine-xanthine phosphoribosyltransferase. J Bacteriol. 1976 Jun;126(3):1141–1148. doi: 10.1128/jb.126.3.1141-1148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irr J., Gallant J. The control of ribonucleic acid synthesis in Escherichia coli. II. Stringent control of energy metabolism. J Biol Chem. 1969 Apr 25;244(8):2233–2239. [PubMed] [Google Scholar]
- Jensen K. F., Houlberg U., Nygaard P. Thin-layer chromatographic methods to isolate 32P-labeled 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP): determination of cellular PRPP pools and assay of PRPP synthetase activity. Anal Biochem. 1979 Oct 1;98(2):254–263. doi: 10.1016/0003-2697(79)90138-6. [DOI] [PubMed] [Google Scholar]
- Levine R. A., Taylor M. W. Regulation of purine savage enzymes in E. coli. Adv Exp Med Biol. 1979;122B:57–60. doi: 10.1007/978-1-4684-8559-2_11. [DOI] [PubMed] [Google Scholar]
- Levine R. A., Taylor M. W. Selection for purine regulatory mutants in an E. coli hypoxanthine phosphoribosyl transferase-guanine phosphoribosyl transferase double mutant. Mol Gen Genet. 1981;181(3):313–318. doi: 10.1007/BF00425604. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. INHIBITION OF THE BIOSYNTHESIS OF THE PYRIMIDINE PORTION OF THIAMINE BY ADENOSINE. J Bacteriol. 1964 Oct;88:1024–1029. doi: 10.1128/jb.88.4.1024-1029.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell P. C., Tucker R. G. Biosynthesis of the pyrimidine moiety of thiamine. A new route of pyrimidine biosynthesis involving purine intermediates. Biochem J. 1968 Jan;106(1):279–287. doi: 10.1042/bj1060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS J. J., BROWN G. M. THE BIOSYNTHESIS OF FOLIC ACID. IV. ENZYMATIC SYNTHESIS OF DIHYDROFOLIC ACID FROM GUANINE AND RIBOSE COMPOUNDS. J Biol Chem. 1964 Jan;239:317–325. [PubMed] [Google Scholar]
- Soska J. Growth of Lactobacillus acidophilus in the absence of folic acid. J Bacteriol. 1966 May;91(5):1840–1847. doi: 10.1128/jb.91.5.1840-1847.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


