Abstract
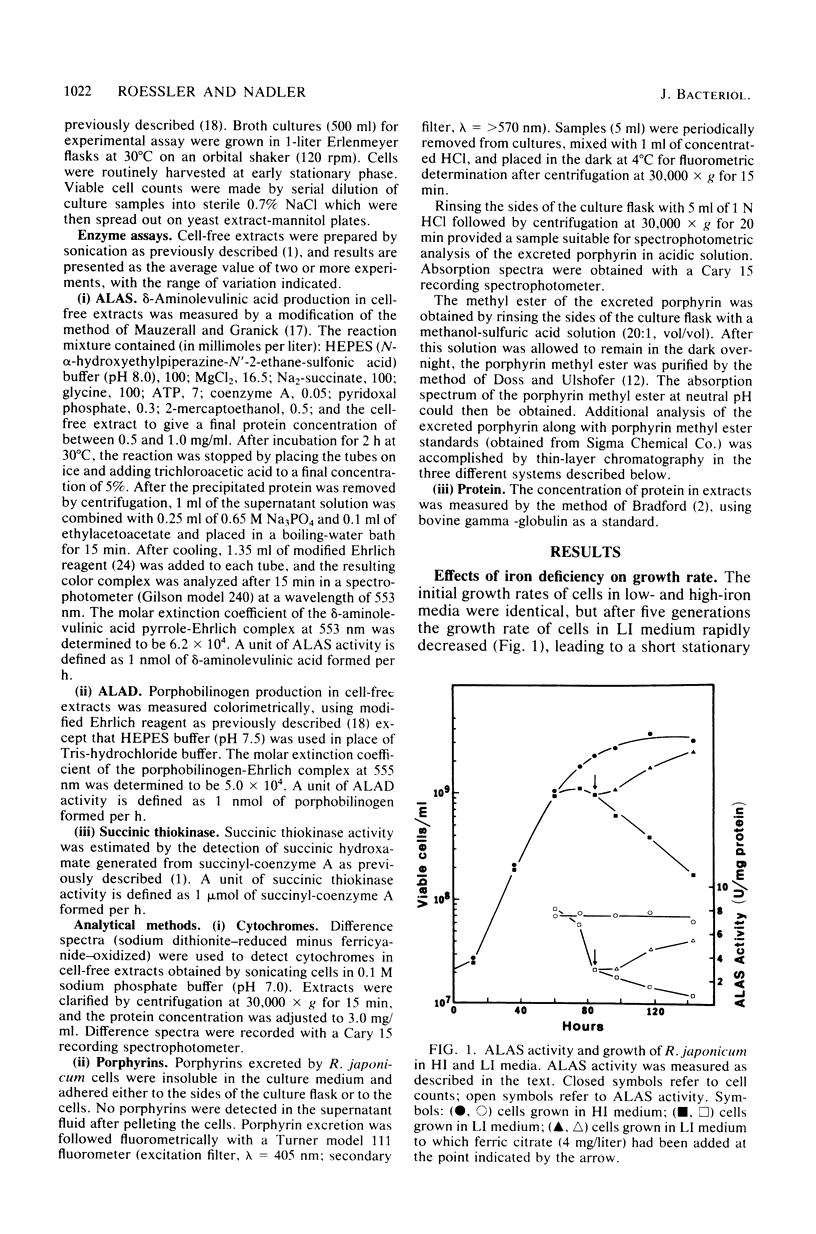
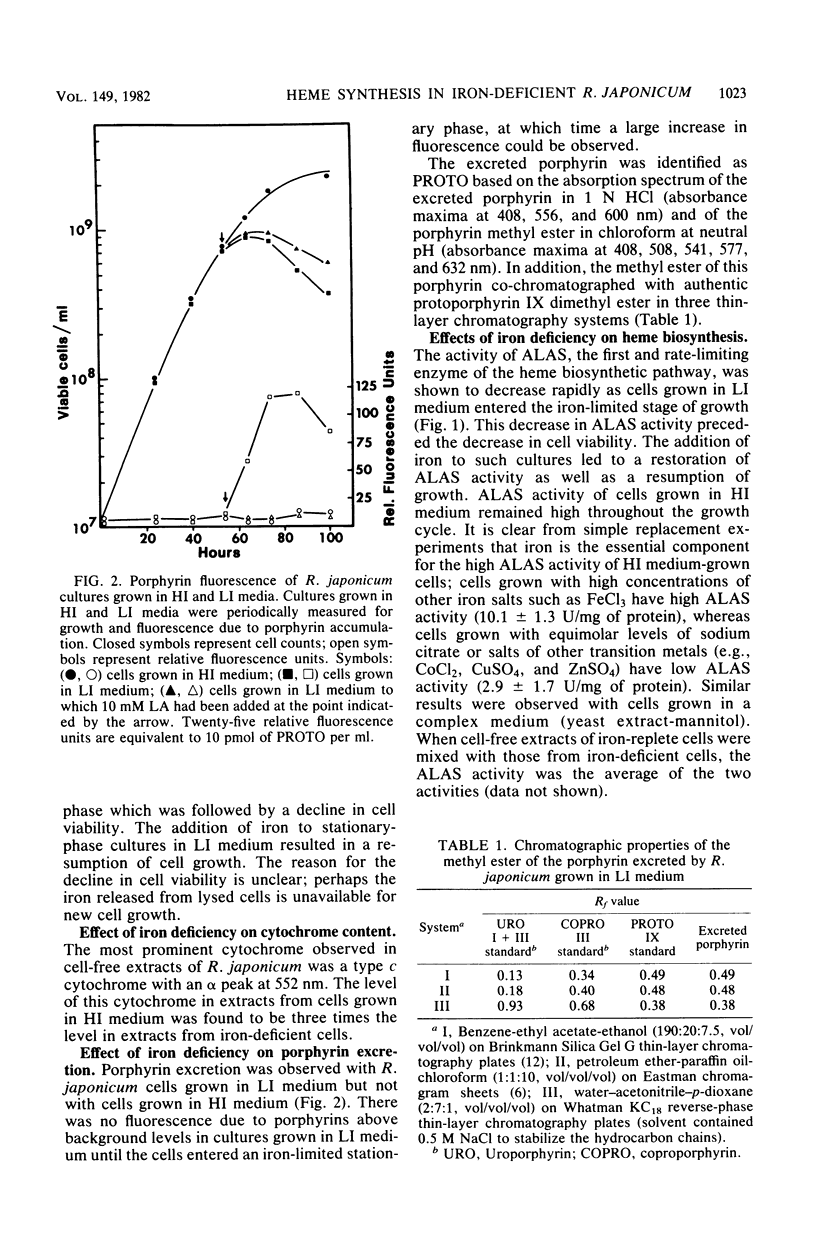
The effects of iron deficiency on heme biosynthesis in Rhizobium japonicum were examined. Iron-deficient cells had a decreased maximum cell yield and a decreased cytochrome content and excreted protoporphyrin into the growth medium. The activities of the first two enzymes of heme biosynthesis, delta-aminolevulinic acid synthase (EC 2.3.1.37) and delta-aminolevulinic acid dehydrase (EC 4.2.1.24), were diminished in iron-deficient cells, but were returned to normal levels upon addition of iron to the cultures. The addition of iron salts, iron chelators, hemin, or protoporphyrin to cell-free extracts did not affect the activity of these enzymes. The addition of levulinic acid to iron-deficient cultures blocked protoporphyrin excretion and also resulted in high delta-aminolevulinic acid synthase and delta-aminolevulinic acid dehydrase activities. These results suggest the possibility that rhizobial heme biosynthesis in the legume root nodule may be affected by the release of iron from the host plant to the bacteroids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Nadler K. D. Stimulation of tetrapyrrole formation in Rhizobium japonicum by restricted aeration. J Bacteriol. 1978 Sep;135(3):782–789. doi: 10.1128/jb.135.3.782-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN E. G. Evidence for the involvement of ferrous iron in the biosynthesis of delta-aminolaevulic acid by chicken erythrocyte preparations. Nature. 1958 Aug 2;182(4631):313–315. doi: 10.1038/182313b0. [DOI] [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chandrika S. R., Padmanaban G. Site of action of iron in the induction of delta-aminolevulinate dehydratase in Neurospora crassa. Biochem Biophys Res Commun. 1977 Jul 11;77(1):35–41. doi: 10.1016/s0006-291x(77)80161-7. [DOI] [PubMed] [Google Scholar]
- Chu T. C., Chu E. J. Thin-layer chromatography of methyl esters of porphyrins, chlorins and related compounds with Eastman "chromagram". J Chromatogr. 1966 Jan;21(1):46–51. doi: 10.1016/s0021-9673(01)91259-2. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Rittenberg B., Lascelles J. Cytochrome synthesis and its regulation in Spirillum itersonii. J Bacteriol. 1967 Nov;94(5):1648–1655. doi: 10.1128/jb.94.5.1648-1655.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The biogenesis of leghemoglobin. The determinant in the Rhizobium-legume symbiosis for leghemoglobin specificity. Biochim Biophys Acta. 1971 Jan 19;229(1):58–62. [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The control of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1971 Feb 28;261(2):321–327. doi: 10.1016/0304-4165(72)90054-2. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969 Dec 30;192(3):486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J. The plant as the genetic determinant of leghaemoglobin production in the legume root nodule. Biochim Biophys Acta. 1969 Jul 30;184(2):432–441. doi: 10.1016/0304-4165(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Doss M., Ulshöfer B. Porphyrin stability as a function of the number of carboxylic acid side chains. Biochim Biophys Acta. 1971 May 18;237(2):356–360. doi: 10.1016/0304-4165(71)90330-8. [DOI] [PubMed] [Google Scholar]
- Godfrey C. A., Dilworth M. J. Haem biosynthesis from ( 14 C)- -aminolaevulinic acid in laboratory-grown and root nodule Rhizobium lupini. J Gen Microbiol. 1971 Dec;69(3):385–390. doi: 10.1099/00221287-69-3-385. [DOI] [PubMed] [Google Scholar]
- Hsu W. P., Miller G. W. Coproporphyrinogenase in tobacco (Nicotiana tabacum L.). Biochem J. 1970 Apr;117(2):215–220. doi: 10.1042/bj1170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Marsh H. V., Evans H. J., Matrone G. Investigations of the Role of Iron in Chlorophyll Metabolism. II. Effect of Iron Deficiency on Chlorophyll Synthesis. Plant Physiol. 1963 Nov;38(6):638–642. doi: 10.1104/pp.38.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K. D., Avissar Y. J. Heme Synthesis in Soybean Root Nodules: I. On the Role of Bacteroid delta-Aminolevulinic Acid Synthase and delta-Aminolevulinic Acid Dehydrase in the Synthesis of the Heme of Leghemoglobin. Plant Physiol. 1977 Sep;60(3):433–436. doi: 10.1104/pp.60.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi D. L., Shemin D. Delta-aminolevulinic acid dehydratase of Rhodopseudomonas spheroides. 3. Mechanism of porphobilinogen synthesis. J Biol Chem. 1968 Mar 25;243(6):1236–1242. [PubMed] [Google Scholar]
- Neuberger A., Sandy J. D., Tait G. H. Control of 5-aminolaevulinate synthetase activity in Rhodopseudomonas spheroides. The involvement of sulphur metabolism. Biochem J. 1973 Nov;136(3):477–490. doi: 10.1042/bj1360477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger A., Sandy J. D., Tait G. H. Control of 5-aminolaevulinate synthetase activity in Rhodopseudomonas spheroides. The purification and properties of an endogenous activator of the enzyme. Biochem J. 1973 Nov;136(3):491–499. doi: 10.1042/bj1360491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]


