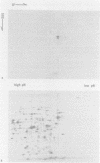
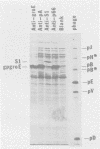
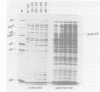
Abstract
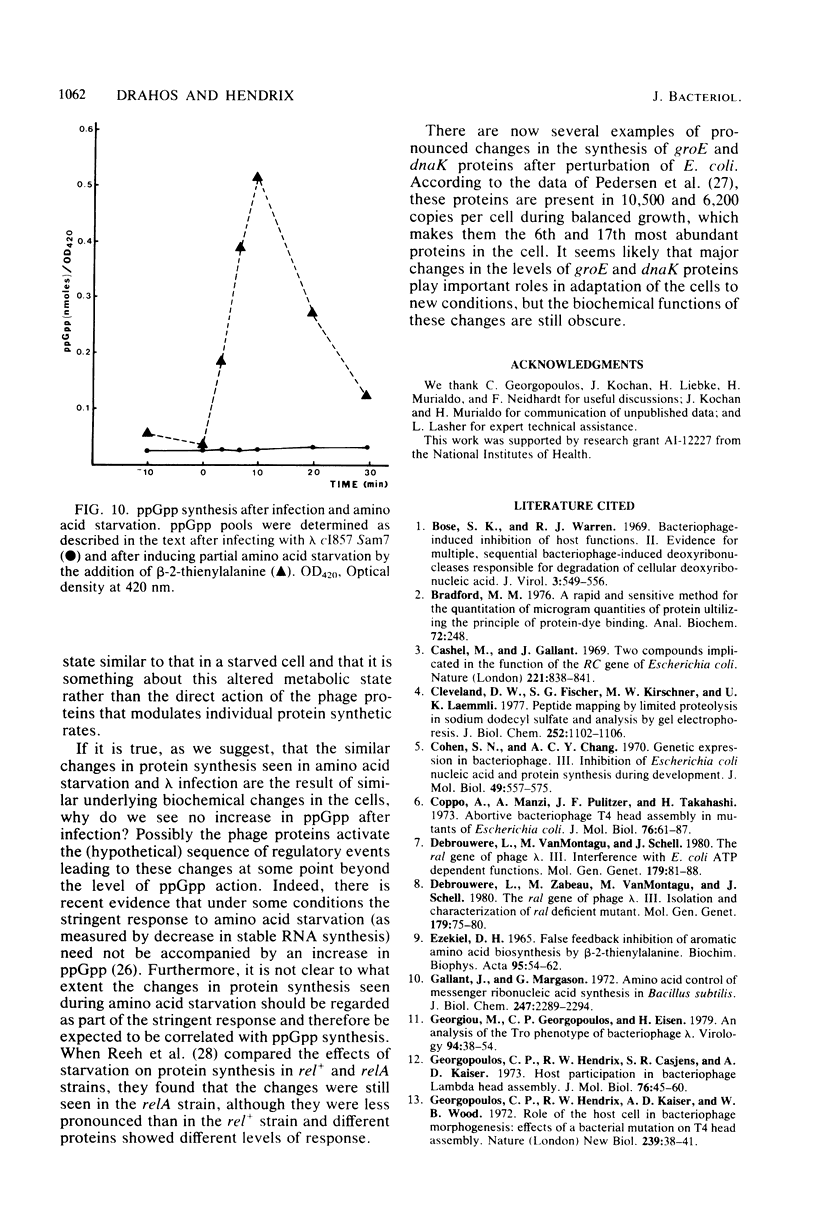
We used two-dimensional gel electrophoresis to quantitate the changes in rates of synthesis that follow phage lambda infection for 21 Escherichia coli proteins, including groE and dnaK proteins. Although total protein synthesis and the rates of synthesis of most individual E. coli proteins decreased after infection, some proteins, including groE protein, dnaK protein, and stringent starvation protein, showed increases to rates substantially above their preinfection rates. Infection by lambda Q- affected host synthesis in the same way as infection by gamma+, whereas infection by lambda N- showed no detectable effect on host synthesis. Deletion of the early genes between att and N abolished the effect, and shorter deletions in this region gave intermediate effects. By this sort of deletion mapping, we show that a large part, though not all, of the effect of lambda infection on host protein synthesis can be ascribed to the early region that contains phage genes Ea10 and ral. We compared the changes in protein synthesis after infection with the changes that occur in uninfected cells upon heat shock or amino acid starvation. The spectrum of changes that occurred on infection was very different from that seen after heat shock but quite similar to that seen during amino acid starvation. Despite this similarity of the effects of lambda infection and starvation, we did not detect any increase in the level of guanosine tetraphosphate during infection. We show that the groE protein is the same protein as B56.5 of Lemaux et al. (Cell 13:427-434, 1978) and A protein of Subramanian et al. (Eur. J. Biochem. 67:591-601, 1976).
Full text
PDF




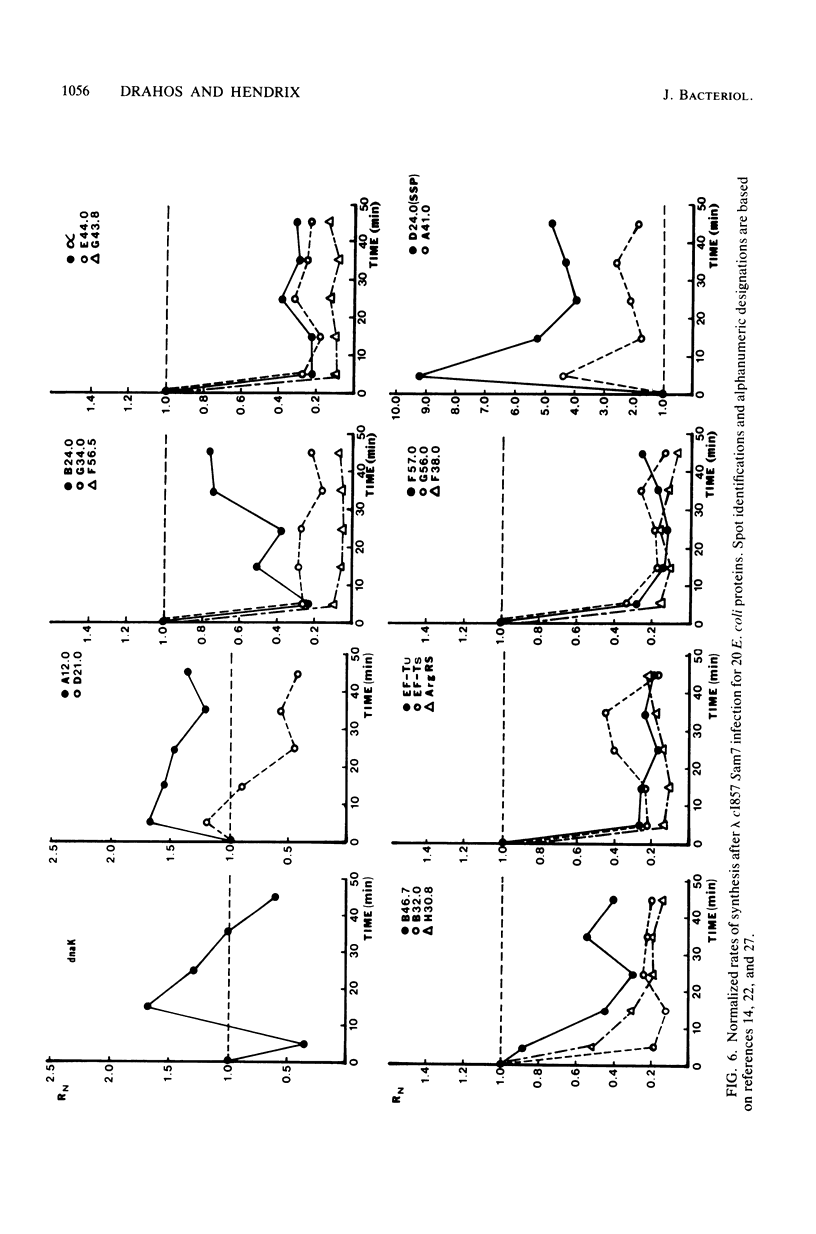

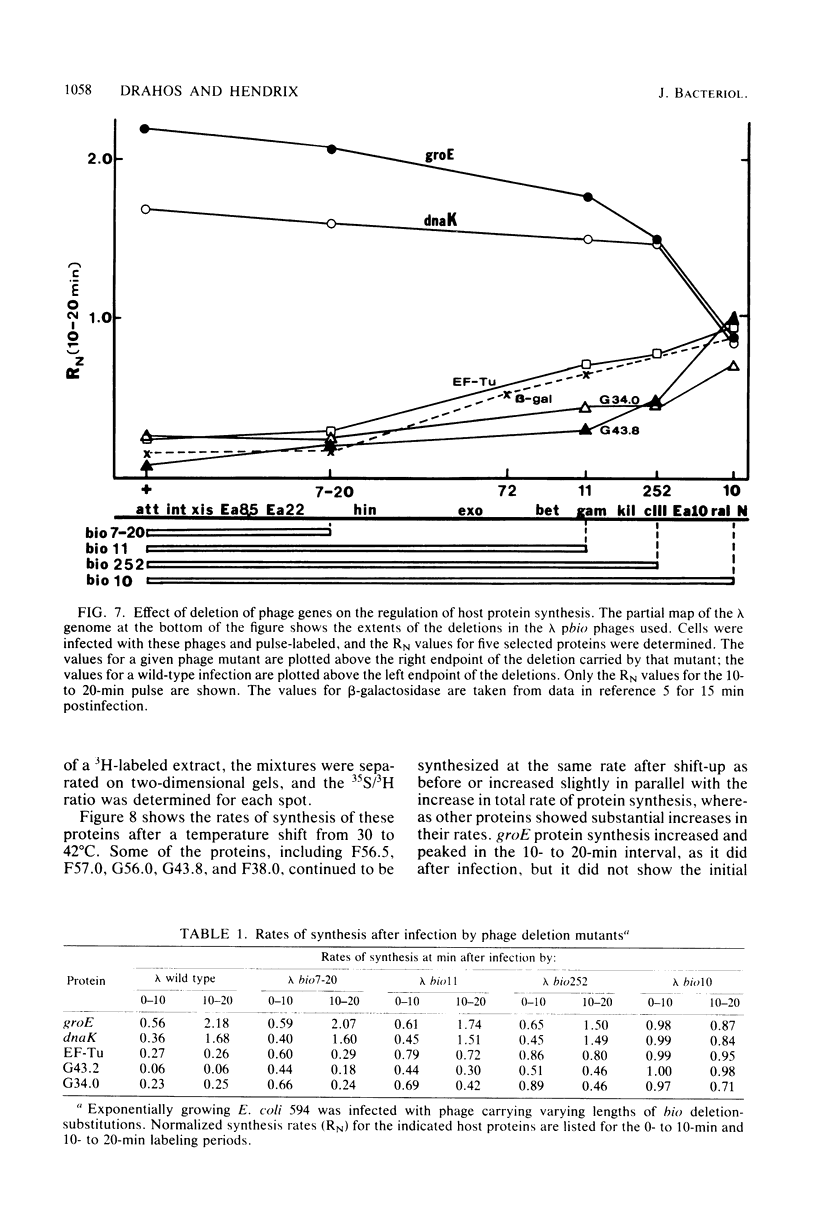
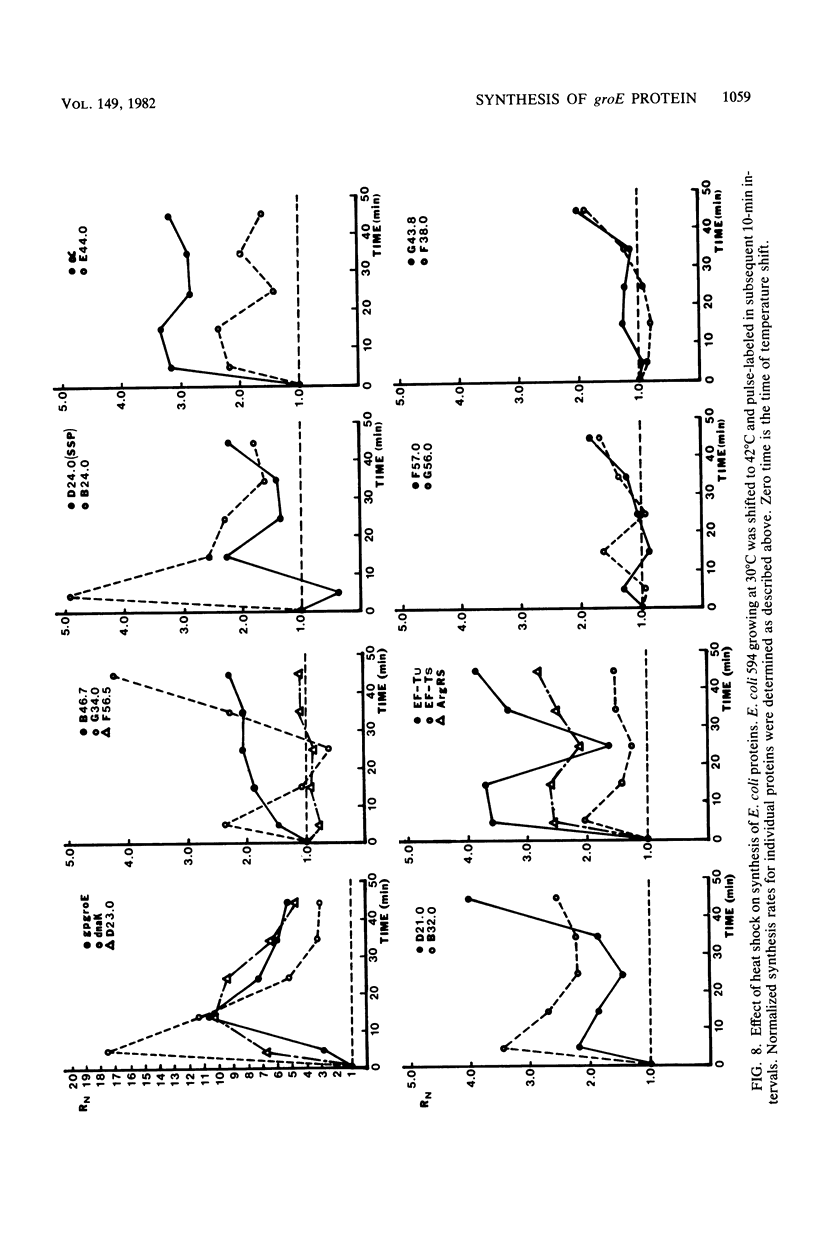

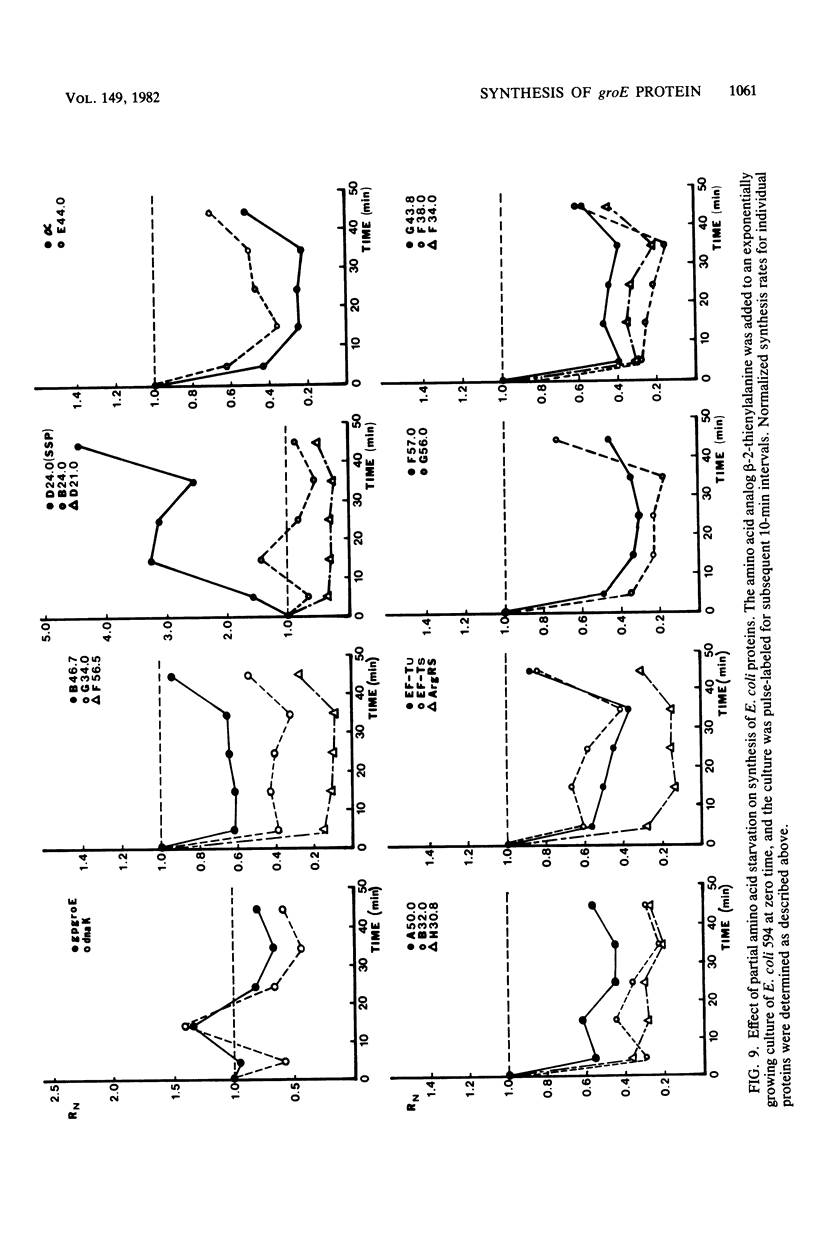

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bose S. K., Warren R. J. Bacteriophage-induced inhibition of host functions. II. Evidence for multiple, sequential bacteriophage-induced deoxyribonucleases responsible for degradation of cellular deoxyribonucleic acid. J Virol. 1969 Jun;3(6):549–556. doi: 10.1128/jvi.3.6.549-556.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Genetic expression in bacteriophage lambda. 3. Inhibition of Escherichia coli nucleic acid and protein synthesis during lambda development. J Mol Biol. 1970 May 14;49(3):557–575. doi: 10.1016/0022-2836(70)90281-0. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Abortive bacteriophage T4 head assembly in mutants of Escherichia coli. J Mol Biol. 1973 May 5;76(1):61–87. doi: 10.1016/0022-2836(73)90081-8. [DOI] [PubMed] [Google Scholar]
- Debrouwere L., Van Montagu M., Schell J. The ral gene of phage lambda. III. Interference with E. coli ATP dependent functions. Mol Gen Genet. 1980;179(1):81–88. doi: 10.1007/BF00268449. [DOI] [PubMed] [Google Scholar]
- Debrouwere L., Zabeau M., Van Montagu M., Schell J. The ral gene of phage lambda. II. Isolation and characterization of ral deficient mutants. Mol Gen Genet. 1980;179(1):75–80. doi: 10.1007/BF00268448. [DOI] [PubMed] [Google Scholar]
- EZEKIEL D. H. FALSE FEEDBACK INHIBITION OF AROMATIC AMINO ACID BIOSYNTHESIS BY BETA-2-THIENYLALANINE. Biochim Biophys Acta. 1965 Jan 11;95:54–62. doi: 10.1016/0005-2787(65)90210-8. [DOI] [PubMed] [Google Scholar]
- Gallant J., Margason G. Amino acid control of messenger ribonucleic acid synthesis in Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2289–2294. [PubMed] [Google Scholar]
- Georgiou M., Georgopoulos C. P., Eisen H. An analysis of the Tro phenotype of bacteriophage lambda. Virology. 1979 Apr 15;94(1):38–54. doi: 10.1016/0042-6822(79)90436-7. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Kaiser A. D., Wood W. B. Role of the host cell in bacteriophage morphogenesis: effects of a bacterial mutation on T4 head assembly. Nat New Biol. 1972 Sep 13;239(89):38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C., Tilly K., Drahos D., Hendrix R. The B66.0 protein of Escherichia coli is the product of the dnaK+ gene. J Bacteriol. 1982 Mar;149(3):1175–1177. doi: 10.1128/jb.149.3.1175-1177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Tsui L. Role of the host in virus assembly: cloning of the Escherichia coli groE gene and identification of its protein product. Proc Natl Acad Sci U S A. 1978 Jan;75(1):136–139. doi: 10.1073/pnas.75.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S., Kronvall G. The use of protein A-containing Staphylococcus aureus as a solid phase anti-IgG reagent in radioimmunoassays as exemplified in the quantitation of alpha-fetoprotein in normal human adult serum. Eur J Immunol. 1974 Jan;4(1):29–33. doi: 10.1002/eji.1830040108. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kiger J. A., Jr, Young E. T., 2nd, Sinsheimer R. L. Purification and properties of intracellular lamba DNA rings. J Mol Biol. 1968 Apr 28;33(2):395–413. doi: 10.1016/0022-2836(68)90197-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Phillips T. A., VanBogelen R. A., Smith M. W., Georgalis Y., Subramanian A. R. Identity of the B56.5 protein, the A-protein, and the groE gene product of Escherichia coli. J Bacteriol. 1981 Jan;145(1):513–520. doi: 10.1128/jb.145.1.513-520.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pao C. C., Dyess B. T. Stringent control of RNA synthesis in the absence of guanosine 5'-diphosphate-3'-diphosphate. J Biol Chem. 1981 Mar 10;256(5):2252–2257. [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Reeh S., Pedersen S., Friesen J. D. Biosynthetic regulation of individual proteins in relA+ and relA strains of Escherichia coli during amino acid starvation. Mol Gen Genet. 1976 Dec 22;149(3):279–289. doi: 10.1007/BF00268529. [DOI] [PubMed] [Google Scholar]
- Schleif R., Greenblatt J., Davis R. W. Dual control of arabinose genes on transducing phage lambda-dara. J Mol Biol. 1971 Jul 14;59(1):127–150. doi: 10.1016/0022-2836(71)90417-7. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). I. Initial characterization. J Mol Biol. 1973 May 5;76(1):1–23. doi: 10.1016/0022-2836(73)90078-8. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). II. The propagation of phage lambda. J Mol Biol. 1973 May 5;76(1):25–44. doi: 10.1016/0022-2836(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Haase C., Giesen M. Isolation and characterization of a growth-cycle-reflecting, high-molecular-weight protein associated with Escherichia coli ribosomes. Eur J Biochem. 1976 Aug 16;67(2):591–601. doi: 10.1111/j.1432-1033.1976.tb10725.x. [DOI] [PubMed] [Google Scholar]
- Szybalski E. H., Szybalski W. A comprehensive molecular map of bacteriophage lambda. Gene. 1979 Nov;7(3-4):217–270. doi: 10.1016/0378-1119(79)90047-7. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Coppo A., Manzi A., Martire G., Pulitzer J. F. Design of a system of conditional lethal mutations (tab/k/com) affecting protein-protein interactions in bacteriophage T4-infected Escherichia coli. J Mol Biol. 1975 Aug 25;96(4):563–578. doi: 10.1016/0022-2836(75)90139-4. [DOI] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Tilly K., Murialdo H., Georgopoulos C. Identification of a second Escherichia coli groE gene whose product is necessary for bacteriophage morphogenesis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1629–1633. doi: 10.1073/pnas.78.3.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentin L. P., Hasnain S., Gallin W. Ribosomal protein S1/S1A in bacteria. FEBS Lett. 1977 Jul 15;79(2):258–263. doi: 10.1016/0014-5793(77)80799-0. [DOI] [PubMed] [Google Scholar]
- Weigle J. Assembly of phage lambda in vitro. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1462–1466. doi: 10.1073/pnas.55.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Temperature-induced synthesis of specific proteins in Escherichia coli: evidence for transcriptional control. J Bacteriol. 1980 Jun;142(3):843–851. doi: 10.1128/jb.142.3.843-851.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabeau M., Friedman S., Van Montagu M., Schell J. The ral gene of phage lambda. I. Identification of a non-essential gene that modulates restriction and modification in E. coli. Mol Gen Genet. 1980;179(1):63–73. doi: 10.1007/BF00268447. [DOI] [PubMed] [Google Scholar]
- Zabeau M., Schell J., Van Montagu M. The alleviation of host-controlled restriction of unmodified phages by functions of bacteriophage lambda. Arch Int Physiol Biochim. 1973 Dec;81(5):990–990. [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]