Abstract
Cells sense and physiologically respond to environmental stress via signaling pathways. Saccharomyces cerevisiae cells respond to cell wall stress by transiently depolarizing the actin cytoskeleton. We report that cell wall stress also induces a transient depolarized distribution of the cell wall biosynthetic enzyme glucan synthase FKS1 and its regulatory subunit RHO1, possibly as a mechanism to repair general cell wall damage. The redistribution of FKS1 is dependent on the actin cytoskeleton. Depolarization of the actin cytoskeleton and FKS1 is mediated by the plasma membrane protein WSC1, the RHO1 GTPase switch, PKC1, and a yet-to-be defined PKC1 effector branch. WSC1 behaves like a signal transducer or a stress-specific actin landmark that both controls and responds to the actin cytoskeleton, similar to the bidirectional signaling between integrin receptors and the actin cytoskeleton in mammalian cells. The PKC1-activated mitogen-activated protein kinase cascade is not required for depolarization, but rather for repolarization of the actin cytoskeleton and FKS1. Thus, activated RHO1 can mediate both polarized and depolarized cell growth via the same effector, PKC1, suggesting that RHO1 may function as a rheostat rather than as a simple on-off switch.
Keywords: actin cytoskeleton, yeast, PKC1, integrins, WSC1
Growth of a Saccharomyces cerevisiae cell is polarized, occurring at a defined position on the cell surface. This oriented growth is mediated by a polarized actin cytoskeleton that directs secretory vesicles to the growth or bud site (Chant 1996; Li et al. 1995; Madden and Snyder 1998). The actin cytoskeleton is composed of two morphologically distinct pools of actin polymers, patches and cables. Patches are foci of actin and actin-binding proteins that are associated with plasma membrane invaginations (Mulholland et al. 1994), and move rapidly over long distances (Waddle et al. 1996). Cables are bundles of overlapping actin filaments (Karpova et al. 1998). Recent studies suggest that patches and cables associate and form an integrated system (Amberg 1998; Karpova et al. 1998). The distribution of actin patches and cables is polarized in a cell cycle–dependent manner (Adams and Pringle 1984; Schmidt and Hall 1998). In G0 cells, actin patches and cables are randomly distributed. As a yeast cell initiates growth, patches concentrate at a preselected bud site, forming a ring structure, and cables orient toward this site. Bud emergence begins and subsequently actin patches migrate into the bud. During bud maturation, patches are found exclusively in the bud, and cables are aligned longitudinally from the mother cell into the bud. As the daughter cell reaches a critical size, the asymmetric distribution of actin is lost. Late in the cell cycle, before cytokinesis, the actin cytoskeleton repolarizes as patches accumulate in and cables orient toward the mother–bud neck region. The cell cycle–dependent organization of the actin cytoskeleton is controlled by Rho-type guanosine triphosphatases (GTPases)1 (Schmidt and Hall 1998).
Five Rho-like GTPases have been identified in budding yeast (CDC42 and RHO1–4), and all have been implicated in the organization of the actin cytoskeleton (Leberer et al. 1997; Helliwell et al. 1998b; Schmidt and Hall 1998; Tanaka and Takai 1998). CDC42 is required for establishing the polarity of the actin cytoskeleton and is, thus, required for emergence of a bud at a preselected site. RHO1–4 further mediate the polarized organization of the actin cytoskeleton, and are required for maintenance of bud growth.
RHO1 mediates bud growth by controlling polarization of the actin cytoskeleton and cell wall synthesis (Cabib et al. 1998). RHO1 is activated by at least two separate inputs, the phosphatidylinositol kinase homologue TOR2 and cell wall defects, both of which activate RHO1 via the exchange factor ROM2 (and possibly also via the related exchange factor ROM1) (Ozaki et al. 1996; Schmidt et al. 1997; Bickle et al. 1998). Cell wall defects, which hyperactivate ROM2 (Bickle et al. 1998), might be sensed and signaled to ROM2 by the plasma membrane protein WSC1/SLG1/HCS77 (see below) (Gray et al. 1997; Verna et al. 1997; Jacoby et al. 1998). RHO1 has four downstream effectors. It binds and activates PKC1, which in turn controls the actin cytoskeleton and transcription of cell wall biosynthesis genes via a mitogen-activated protein (MAP) kinase cascade (Nonaka et al. 1995; Igual et al. 1996; Kamada et al. 1996; Helliwell et al. 1998b; Zhao et al. 1998; see below). RHO1 also binds and activates the integral plasma membrane protein FKS1 (β-1,3-glucan synthase) and, thereby, controls cell wall synthesis directly (Drgonova et al. 1996; Qadota et al. 1996). Finally, RHO1 interacts with BNI1, a formin family member, and SKN7, a two component signaling protein (Kohno et al. 1996; Alberts et al. 1998). RHO1 may also control the actin cytoskeleton via BNI1 that binds profilin (Evangelista et al. 1997). RHO1 and ROM2 are localized at growth sites (Yamochi et al. 1994; Manning et al. 1997), and RHO1 may activate only a subset of its effectors in response to a particular input (Helliwell et al. 1998b).
The RHO1 effector PKC1 activates a MAP kinase cascade composed of BCK1/SLK1 (MAPKKK), MKK1 and MKK2 (MAPKK), and MPK1/SLT2 (MAPK) (Levin et al. 1994; Banuett 1998; Gustin et al. 1998). This MAP kinase cascade is activated by hypotonic or heat shock, and also during periods of polarized cell growth (Davenport et al. 1995; Kamada et al. 1995; Zarzov et al. 1996), and controls actin organization and transcription of cell wall biosynthesis genes (Igual et al. 1996; Helliwell et al. 1998b; Zhao et al. 1998). Heat shock is thought to induce a weakness in the cell wall that leads to activation of the MAP kinase cascade via a putative cell wall sensor (Kamada et al. 1995; Bickle et al. 1998). Several observations suggest that the cell wall sensor is the type I transmembrane protein WSC1/SLG1/HCS77 (Gray et al. 1997; Verna et al. 1997; Jacoby et al. 1998; Lodder et al. 1999). WSC1, a member of the WSC family of plasma membrane proteins that includes WSC1–4 and the related MID2 protein, is required for heat stress activation of the MAP kinase cascade (Gray et al. 1997; Verna et al. 1997; Ketela et al. 1999; Rajavel et al. 1999). Furthermore, a WSC1 deficiency is suppressed by overexpression of ROM2, RHO1, or PKC1 (Gray et al. 1997; Verna et al. 1997; Jacoby et al. 1998).
The yeast cell wall is a dynamic structure that comprises 15–30% of the dry weight of the cell (Orlean 1997; Kapteyn et al. 1999). It is composed of three major components, mannoproteins, β-1,6-glucan, and β-1,3-glucan, all cross-linked to each other and to chitin, an N-acetylglucosamine polymer (Kollar et al. 1997; Kapteyn et al. 1999). β-1,3-glucan is synthesized by the integral plasma membrane protein FKS1. The cell wall determines cell shape, provides rigidity that counteracts the outward turgor pressure on the plasma membrane, and acts as a diffusion barrier delimiting the periplasmic space. Cell wall synthesis, a poorly understood process, is temporally and spatially controlled in response to growth, stress, and mating signals (Orlean 1997; Kapteyn et al. 1999).
The yeast actin cytoskeleton is also reorganized in response to mating pheromone or environmental stress. Mating pheromone induces polarization of the actin cytoskeleton and a characteristic mating projection (Read et al. 1992). These changes are mediated by the well characterized pheromone signaling pathway and the Rho-type GTPase CDC42 (Leberer et al. 1997; Schmidt and Hall 1998; Tanaka and Takai 1998). Hypertonic or heat shock induces a transient depolarization of the actin cytoskeleton (Chowdhury et al. 1992; Lillie and Brown 1994). However, the physiological significance of this response, and the factors or signaling pathways that mediate it are unknown. In mammalian cells, the induction of actin stress fibers is controlled by integrins and Rho GTPases (Hall 1998; Howe et al. 1998; Schmidt and Hall 1998).
Here, we investigate the transient depolarization of the actin cytoskeleton in response to environmental stress. We show that actin depolarization in response to cell wall damage is necessary to depolarize glucan synthase FKS1. The transient depolarization of the actin cytoskeleton and FKS1 may be a homeostasis mechanism to repair general cell wall damage. This response is controlled by a putative signaling pathway consisting of WSC1, ROM2, RHO1, and PKC1. The PKC1-activated MAP kinase pathway is not required for this depolarization process, but rather for repolarization, suggesting that a yet-to-be defined PKC1 effector pathway mediates depolarization.
Material and Methods
Strains, Plasmids, and Media
The S. cerevisiae strains and plasmids used in this study are listed in Table and Table , respectively. All strains are isogenic derivatives of JK9-3d. Rich media (YPD) or synthetic minimal media (SD, SRAFF, and SGAL) complemented with the appropriate nutrients for plasmid maintenance were as described (Guthrie and Fink 1991). Latrunculin-A (200 μM) was added to cultures from a 200-mM stock in DMSO. A culture of cells was treated with Zymolyase 20T (7.5 U/ml; Seikagaku Corp.) added from a 750-U/ml stock in YPD at 30°C for 20 min. A culture of cells was treated with SDS (0.02%) added from a 10% stock in water at 24 or 30°C for 10 min. For all heat shock experiments, 2-ml aliquots were removed from a logarithmic phase culture grown at 24°C and incubated at 37°C for the indicated time.
Table 1.
Yeast Strains Used in This Study
| Strain | Genotype |
|---|---|
| JK9-3da/α | MAT a/α leu2-3,112/leu2-3,112 ura3-52/ura3-52 trp1/trp1 his4/his4 rme1/rme1 HML a /HML a |
| JK9-3da | MAT a leu2-3,112 ura3-52 trp1 his4 rme1 HML a |
| TB50a | MAT a leu2-3,112 ura3-52 trp1 his3 rme1 HML a |
| MH272-1da | MATa leu2-3,112 ura3-52 trp1 his3 rme1 HMLa |
| AS135-1a | JK9-3d a rho2::kanMX4 |
| AS138-1b | JK9-3da rom2::URA3 |
| AS167-1d | MH272-1da bni1::URA3 |
| IH1-5a | TB50a wsc4::HIS3MX6 |
| MB146-3d | MH272-1da fks1::HIS3 |
| NB75-1b | JK9-3da skn7::LEU2 |
| PA39-1b | JK9-3da wsc1::kanMX4 |
| PA62-2d | JK9-3da wsc3::kanMX4 |
| PA88-1a | JK9-3da wsc2::kanMX4 |
| PA96 | JK9-3da/α wsc1::kanMX4/wsc1::kanMX4 |
| PA109-1c | TB50a bck1::HIS3MX6 |
| PA120-3b | JK9-3da rho1::kanMX4 / pHA-RHO1 |
| PE3-2a | JK9-3da mid2::kanMX4 |
| SD4-2c | JK9-3da rom1::kanMX4 |
| TS45-1a | TB50a mpk1::TRP1 |
Table 2.
Plasmids Used in This Study
| Plasmid | Characteristic and source |
|---|---|
| pGAL-BCK1* | pPAD91, BCK1-20 allele under the control of the GAL1 promoter |
| pGAL-MKK1* | pNV7-MKK1(P386), expresses activated-MKK1 under the control of the GAL1 promoter (Watanabe et al. 1995) |
| pGAL-PKC1* | pBM743, expresses activated-PKC1 under the control of the GAL1 promoter (D.E. Levin) |
| pGAL-RHO1 | pTB230, expresses the functional NH2 terminally 2×HA-tagged RHO1 under the control of the GAL1 promoter |
| (Schmidt et al. 1997) | |
| pGAL-RHO1* | pAS106, expresses functional NH2 terminally HA-tagged activated RHO1 (H68) under the control of the GAL1 promoter |
| pGAL-RHO2 | pAS35, expresses functional NH2 terminally HA-tagged RHO2 under the control of the GAL1 promoter |
| pGAL-ROM2 | pAS48, expresses functional NH2 terminally HA-tagged ROM2 under the control of the GAL1 promoter |
| pGAL-WSC1 | pPAD62, WSC1 under the control of the GAL1 promoter |
| pGAL-WSC2 | pPAD45, expresses functional COOH terminally cmyc-tagged WSC2 under the control of the GAL1 promoter |
| pGAL-WSC3 | pPAD48, expresses functional COOH terminally cmyc-tagged WSC3 under the control of the GAL1 promoter |
| pHA-RHO1 | pAS97, expresses functional NH2 terminally HA-tagged RHO1 from its own promoter (CEN URA3) |
| pHAs | pPAD80/81/82/83, 3×HA tagging vector (see Materials and Methods), |
| 2μLEU2, CEN LEU2, CEN URA3, 2μURA3, respectively | |
| pWSC1-HA | pPAD86, expresses functional COOH terminally 3×HA-tagged WSC1 from its own promoter (see Materials and Methods) |
Genetic Techniques
Plasmid DNA was isolated as described (Sambrook et al. 1989). Yeast transformation was performed by the lithium acetate procedure (Ito et al. 1983). Escherichia coli strain MH1 was used for propagation and isolation of plasmids (Sambrook et al. 1989). The entire open reading frames of WSC1, WSC2, WSC3, BCK1, and MKK1 were replaced by PCR-generated kanMX4 or HIS3MX6 cassettes, as described (Wach et al. 1994). Disruptions were verified by PCR.
Construction of 3×HA-tagged WSC1
A 0.45-kb fragment encoding a triple hemagglutinin (HA) tag followed by the CYC1 transcription terminator was generated by PCR using the plasmid pYADE4 (Brunelli and Pall 1993), containing the CYC1 terminator sequence, as a template and the following two oligonucleotides: 5′-ACATGCATGC TACCCATACGATGTTCCTGACTATGCGGGC-TATCCCTATGACGTCCCGGACTATGCAGGATATCCATATGA-CGTTCCAGATTACGCTTAACCAAGATGGCCTTTGGTGGGTTG- AAGAAG-3′ (SphI site in bold and HA epitope coding sequence underlined) and 5′-ATAGCAAAGATTGAATAAGGC-3′. The 0.45-kb fragment was cut with SphI and HindIII, and the resulting fragment was cloned into YEplac181 (2μLEU2), YCplac111 (CEN LEU2), YCplac33 (CEN URA3), and YEplac195 (2μURA3) (Gietz and Sugino 1988), creating plasmids pPAD80, pPAD81, pPAD82, and pPAD83, respectively. These plasmids, containing a 3×HA coding sequence preceded by a multiple cloning site and followed by a termination codon and the CYC1 terminator sequence, are useful for constructing COOH terminally 3×HA–tagged proteins. pPAD86 (pPAD81::WSC1) (CEN LEU2) contains WSC1 as a 1.6-kb SacI-SmaI PCR fragment. The SacI site was introduced 460 nucleotides upstream of the WSC1 initiation codon, and the SmaI site replaced the termination codon.
Indirect Immunofluorescence
Logarithmically growing cells were fixed for 2 h in the growth medium supplemented with formaldehyde (3.7%) and potassium phosphate buffer (100 mM, pH 6.5). Cells were washed and resuspended in sorbitol buffer (1.2 M sorbitol, 100 mM potassium phosphate, pH 6.5). Cell walls were digested for 60–120 min at 37°C in sorbitol buffer supplemented with β-mercaptoethanol (20 mM) and Zymolyase 20T (0.1 mg/ml; Seigagaku Corp.) or lyticase (gift from P. Ernst, Biozentrum Basel). Spheroblasts were attached on poly-l-lysine–coated glass slides and permeabilized in PBT (53 mM Na2HPO4, 13 mM NaH2PO4, 75 mM NaCl, 0.1% Triton X-100). Immunofluorescent detection of FKS1 was performed with the T2B8 anti–FKS1 mAb (Qadota et al. 1996). HA-tagged WSC1 and RHO1 were detected with a high affinity monoclonal anti–HA antibody (clone 16B12; BAbCO) at a final dilution of 1:1,000 in PBT. The samples were treated with primary antibody for 2 h and subsequently with Cy3-conjugated rabbit anti–mouse IgG (Molecular Probes, Inc.), diluted 1:1,000 in PBT for 90 min. Washed cells were examined with a Zeiss Axiophot microscope (100× objective) and a video imaging system (MWG Biotech).
Actin Staining
Logarithmically growing cells were fixed in formaldehyde (3.7%) and potassium phosphate buffer (100 mM, pH 6.5), and stained with TRITC-phalloidin (Sigma Chemical Co.) to visualize actin, as described previously (Benedetti et al. 1994).
Results
Cell Wall Stress Induces Actin Cytoskeleton Depolarization
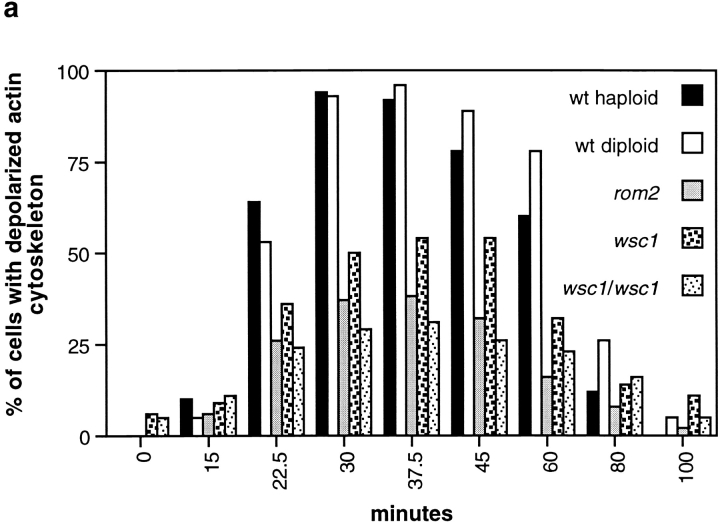
The actin cytoskeleton is transiently depolarized upon heat shock (Lillie and Brown 1994; Desrivieres et al., 1998). To investigate this effect further, we examined the actin cytoskeleton in wild-type cells shifted from 24°C to 37°C for different time intervals (see Materials and Methods). The percentage of small- and medium-budded cells (cells that normally exhibit a polarized actin cytoskeleton) containing a depolarized actin cytoskeleton was determined. Cells containing <50% of their actin patches in the bud were considered to have a depolarized actin cytoskeleton. The maximum percentage of depolarized cells (∼95%) was reached after ∼30 min of heat shock, with complete repolarization occurring after ∼120 min (Fig. 1). The maximally depolarized state persisted for ∼15 min. Thus, as seen previously, heat shock induces a rapid and severe, but transient, depolarization of the actin cytoskeleton. Interestingly, heat shock did not depolarize actin patches at the mother–bud neck in large-budded cells.
Figure 1.
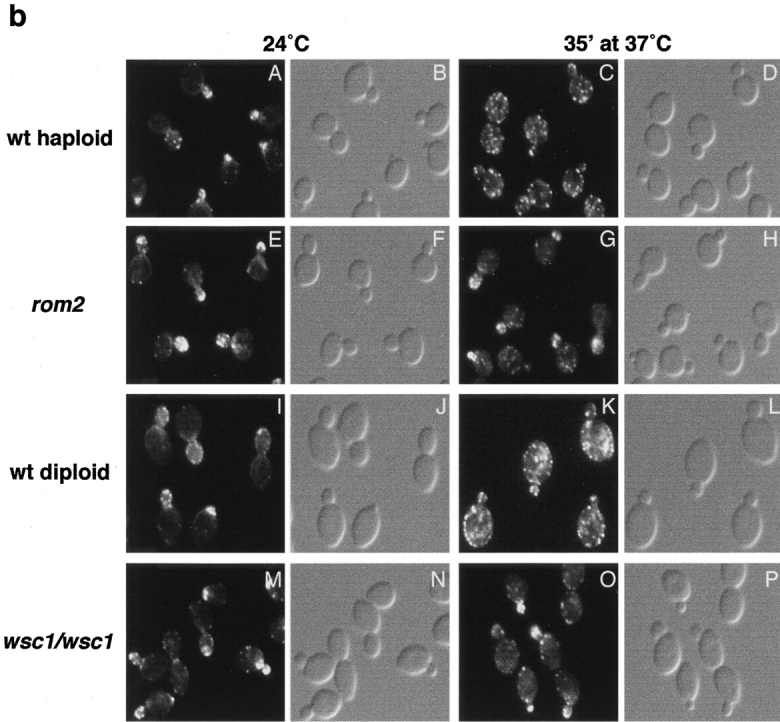
WSC1 and ROM2 are required for heat-induced depolarization of the actin cytoskeleton. (a) Wild-type (wt) (JK9-3da), rom2 (AS138-1b), and wsc1 (PA39-1b) haploid cells, and wild-type (JK9-3da/α) and wsc1/wsc1 (PA96) diploid cells were grown in rich medium at 24°C, shifted to 37°C for the indicated time (in minutes), fixed, and processed for visualization of the actin cytoskeleton. The percentage of cells (n > 200) exhibiting depolarized actin patches was determined (see Materials and Methods). A representative of three experiments is shown. (b) Wild-type (JK9-3da) (A–D) and rom2 (AS138-1b) (E–H) haploid cells, and wild-type (JK9-3da/α) (I–L) and wsc1/wsc1 (PA96) (M-P) diploid cells were grown in rich medium at 24°C, shifted to 37°C for 35 min, fixed, stained with TRITC-phalloidin, and observed by fluorescence (A, C, E, G, I, K, M, and O) and Nomarski (B, D, F, H, J, L, N, and P) microscopy to visualize the actin cytoskeleton and whole cells, respectively.
Heat shock is thought to cause a cell wall weakening (Kamada et al. 1995). To determine if heat-induced actin depolarization is a consequence of a cell wall defect, we examined if osmotic stabilization of the cell wall by 1 M sorbitol blocked the actin depolarization response. The response was partly blocked in cells shifted to a high temperature in the presence of 1 M sorbitol (data not shown). To determine further if actin depolarization is a consequence of a cell wall defect, the actin cytoskeleton was examined in cells in which a cell wall defect was induced either by mild treatment with Zymolyase (β-1,3-glucanase), which digests the cell wall, or by treatment with the anionic detergent SDS (see Materials and Methods) (Kitamura et al. 1974; Igual et al. 1996; Bickle et al. 1998). Essentially all cells treated with 7.5 U/ml Zymolyase or with 0.02% SDS exhibited a depolarized actin cytoskeleton (data not shown). However, unlike heat-induced depolarization, the Zymolyase- and SDS-induced depolarization persisted for the duration of the experiment (120 min), possibly because cells cannot adapt to these treatments as they can adapt to heat shock. These results suggest that depolarization of the actin cytoskeleton is a response to cell wall stress.
Cell Wall Stress Depolarizes the Cellular Distribution of Glucan Synthase
The above findings suggested that cells might delocalize the actin cytoskeleton as a homeostasis mechanism to repair cell wall damage. To investigate this possibility, we first examined the cellular localization of FKS1 in heat shocked cells by immunofluorescence (see Materials and Methods). FKS1, the presumed catalytic subunit of the cell wall biosynthetic enzyme β-1,3-glucan synthase, normally localizes with its regulatory subunit, RHO1, and actin patches at sites of cell growth (Drgonova et al. 1996; Qadota et al. 1996). As expected, FKS1 displayed a polarized distribution in cells growing at 24°C, found associated with the plasma membrane exclusively in the bud. Upon heat shock, the distribution of FKS1 was transiently depolarized, with a time course similar to that of the actin cytoskeleton (Fig. 2 a). After 35 min at 37°C, the FKS1 signal decreased in the bud and appeared uniformly at the periphery of the entire cell. After 120 min at 37°C, FKS1 was relocalized exclusively to the bud. A depolarization of FKS1 was also observed in SDS-treated cells (data not shown). We also examined the cellular localization of the glucan synthase regulatory subunit RHO1 in heat shocked cells. Cells expressing HA-tagged RHO1 (PA120-3b) were shifted from 24 to 37°C and further incubated for 35 or 120 min. Visualization of RHO1 by indirect immunofluorescence revealed a transient depolarization similar to that observed for FKS1 (Fig. 2 b). Thus, glucan synthase is redistributed evenly throughout the cell periphery in response to cell wall stress. This depolarization of cell growth may be to repair general cell wall damage.
Figure 2.
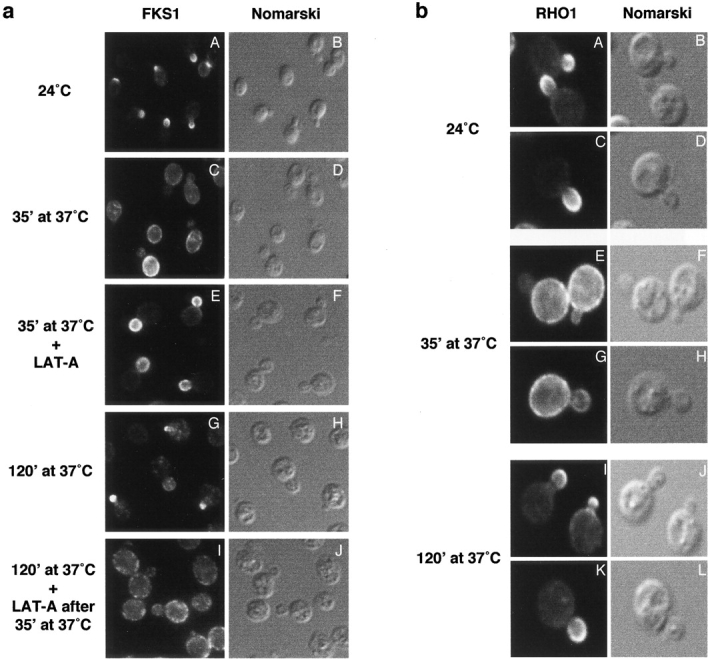
The actin cytoskeleton is required for de- and repolarization of glucan synthase upon heat shock. (a) Wild-type cells (JK9-3da) were grown at 24°C, shifted at 37°C for the indicated time, fixed, and processed for detection of FKS1 by immunofluorescence (see Materials and Methods). LAT-A was added at time of shift to 37°C (E–F) and after 35 min at 37°C (I–J). Fluorescence (A, C, E, G, and I, FKS1) and Nomarski (B, D, F, H, and J) images are shown. (b) Cells expressing HA-RHO1 (PA120-3b) were grown in rich medium at 24°C, shifted to 37°C for the indicated time, fixed, and processed for detection of HA-RHO1 by immunofluorescence (see Materials and Methods). Fluorescence (A, C, E, G, I, and K, RHO1) and Nomarski (B, D, F, H, J, and L) images are shown.
FKS1 Requires the Actin Cytoskeleton for De- and Repolarization
To investigate further the possibility that actin delocalization is part of a homeostasis mechanism to depolarize cell growth and repair general cell wall damage, we examined if FKS1 de- and repolarization are dependent on the actin cytoskeleton. To determine if the actin cytoskeleton is required for FKS1 depolarization, FKS1 localization was examined in cells treated simultaneously with heat and latrunculin-A (LAT-A), a toxin that disrupts the actin cytoskeleton by sequestering actin monomers (Coue et al. 1987). LAT-A prevented the heat-induced depolarization of FKS1, indicating that the actin cytoskeleton is required for FKS1 depolarization (Fig. 2 a). To determine if the actin cytoskeleton is also required for FKS1 repolarization, we examined the effect of LAT-A on depolarized FKS1. Cells previously incubated at a high temperature (37°C) for 35 min were treated with LAT-A and examined for localization of FKS1. After 120 min of heat shock and 85 min of LAT-A treatment, FKS1 remained depolarized, whereas FKS1 repolarized in mock-treated cells (Fig. 2 a). Thus, the actin cytoskeleton is required for both FKS1 de- and repolarization, suggesting that cells depolarize their actin cytoskeleton in response to cell wall damage as a mechanism to depolarize cell growth and repair general cell wall damage.
ROM2 and WSC1, But Not the PKC1-activated MAP Kinase Cascade, Are Required for Stress-induced Actin Cytoskeleton and FKS1 Depolarization
RHO1 and the PKC1-activated MAP kinase cascade are activated by cell wall damage (Kamada et al. 1995; Bickle et al. 1998). To determine if RHO1 or the PKC1-activated MAP kinase cascade mediates the stress-induced depolarization of the actin cytoskeleton, we examined this response in null mutants individually lacking a WSC family member (WSC1, WSC2, WSC3, WSC4, or MID2), a RHO1 exchange factor (ROM1 or ROM2), the nonessential GTPase RHO2, a RHO1 effector (BNI1, SKN7, or FKS1), or a member of the PKC1-activated MAP kinase pathway (BCK1 or MPK1). rho1 and pkc1 null mutants were not examined because RHO1 and PKC1 are essential for growth. The wsc1, wsc2, wsc3, wsc4, mid2, rom1, rom2, rho2, bni1, skn7, fks1, bck1, and mpk1 null mutants were grown at 24°C, shifted to 37°C for different time intervals, and processed for visualization of the actin cytoskeleton. The wsc1 and rom2 mutants were severely, but not completely, defective in heat-induced depolarization of the actin cytoskeleton (Fig. 1). A maximum of ∼55% and ∼40% of the wsc1 and rom2 cells (versus ∼95% for wild-type cells), respectively, exhibited a depolarized actin cytoskeleton after 30 min at 37°C. This defect was not simply a delay in depolarization of the actin cytoskeleton, as a higher percentage of depolarized cells was not observed at later time points. Furthermore, wsc1 and rom2 mutants were sensitive to SDS for growth, and exhibited a defect in FKS1 depolarization that correlated with these mutants' defect in actin depolarization (data not shown). Because a wsc1/wsc1 homozygous diploid strain has a more severe growth defect than a wsc1 haploid (Gray et al. 1997), we also examined the actin cytoskeleton in a heat shocked wsc1/wsc1 diploid. A maximum of ∼30% of the wsc1/wsc1 cells (versus ∼95% for wild-type diploid cells) exhibited a depolarized actin cytoskeleton, after 30 min at 37°C, indicating that the block in depolarization of the actin cytoskeleton was indeed more pronounced in the homozygous diploid mutant. Surprisingly, all of the other mutants listed above exhibited a wild-type–like depolarization response. These observations suggest that stress-induced actin and growth depolarization requires WSC1 and ROM2, but not the PKC1-activated MAPK kinase pathway or any RHO1 effector other than PKC1.
Hyperactivated RHO1 or PKC1, But Not BCK1 or MKK1, Is Sufficient to Induce Actin and FKS1 Depolarization
The previous observation that cell wall defects increase ROM2 exchange activity toward RHO1 (Bickle et al. 1998) and the above finding that a wsc1 or rom2 mutation reduces stress-induced depolarization suggest that the depolarization process requires RHO1 hyperactivation. To examine this further, we investigated the effect of overexpressing constitutively activated RHO1 (RHO1*) on the actin cytoskeleton and FKS1 localization. As RHO1* is toxic, we constructed a plasmid-borne RHO1* allele under control of the strong and galactose-inducible GAL1 promoter (pGAL-RHO1*). This construct was not toxic in cells grown on a carbon source other than galactose (data not shown). Cells containing pGAL-RHO1* were grown to logarithmic phase in medium containing raffinose as a carbon source, and galactose was added to a final concentration of 2%. Aliquots of cells were removed at different time points after addition of galactose and processed for visualization of the actin cytoskeleton. In raffinose-containing medium, cells displayed a normal vegetative distribution of actin patches. After 1.5 h in galactose, the number of patches in the mother cell increased (data not shown). After 2.5 h in galactose, the actin cytoskeleton was depolarized in essentially all cells (Fig. 3). FKS1 was also depolarized, with similar kinetics and severity as observed for the actin cytoskeleton (data not shown). Expression of wild-type RHO1 from the GAL1 promoter (pGAL-RHO1) had no depolarizing effect (data not shown). Thus, RHO1 hyperactivation is sufficient to induce actin and FKS1 depolarization. This finding, combined with the previous findings that cell wall stress hyperactivates ROM2 exchange activity toward RHO1 (Bickle et al. 1998) and that a rom2 mutation blocks depolarization, suggests that depolarization results from RHO1 hyperactivation.
Figure 3.
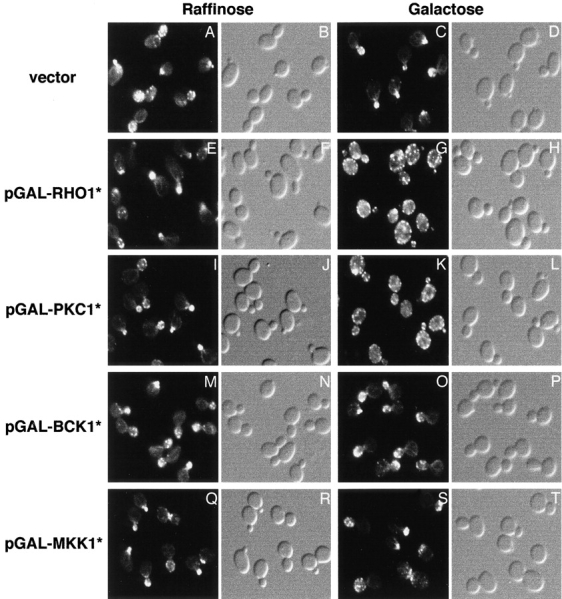
Hyperactivated RHO1 or PKC1 is sufficient to induce actin depolarization. Wild-type (JK9-3da) cells carrying an empty vector (A–D), pGAL-RHO1* (E–H), pGAL-PKC1* (I–L), pGAL-BCK1* (M–P), or pGAL-MKK1* (Q–T) were grown at 30°C in minimal medium containing raffinose. Galactose was added to a final concentration of 2% and strains were further incubated for 2.5 h (empty vector, pGAL-RHO1* or pGAL-PKC1*) or 5 h (pGAL-BCK1* or pGAL-MKK1*). Cells containing pGAL-BCK1* or pGAL-MKK1* gave the same result when incubated in galactose for 2.5 or 5 h. Samples were fixed, stained with TRITC-phalloidin, and observed by fluorescence (A, C, E, G, I, K, M, O, Q, and S) and Nomarski (B, D, F, H, J, L, N, P, R, and T) microscopy to visualize the actin cytoskeleton and whole cells, respectively.
We examined the effect of overexpressed and constitutively activated PKC1 (PKC1*), BCK1 (BCK1*), and MKK1 (MKK1*) on the actin cytoskeleton and FKS1 distribution. Like pGAL-RHO1*, pGAL-PKC1*, pGAL-BCK1*, and pGAL-MKK1* were also toxic uniquely in cells grown on galactose-containing medium (data not shown). The effect of pGAL-PKC1* on the actin cytoskeleton and FKS1 distribution was indistinguishable from that of pGAL-RHO1*, indicating that hyperactivation of PKC1 is also sufficient to elicit the depolarization response (Fig. 3 and data not shown). Hyperactivation of BCK1 or MKK1 did not induce a depolarization of the actin cytoskeleton or FKS1 in cells shifted to galactose for 2.5 (as performed above for RHO1* and PKC1*) or 5 h (Fig. 3 and data not shown). These results further indicate that the PKC1-activated MAP kinase cascade is not involved in stress-induced depolarization of growth, and suggest that a yet-to-be defined PKC1 effector pathway (Lee and Levin 1992; Helliwell et al. 1998b) mediates the depolarization response. Expression of wild-type WSC1, WSC2, WSC3, RHO2, or ROM2 from the GAL1 promoter had no effect on the actin cytoskeleton (data not shown).
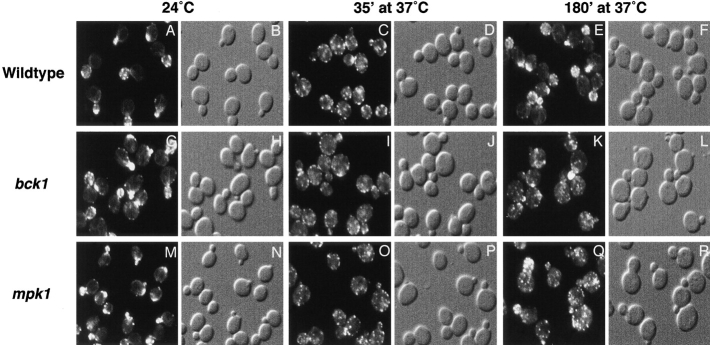
PKC1-activated MAP Kinase Cascade Is Required for Repolarization of the Actin Cytoskeleton and FKS1
The previous findings that mutants defective in MPK1/SLT2 exhibit a depolarized actin cytoskeleton (Mazzoni et al. 1993) and overexpression of MPK1 suppresses the actin polarization defect of a rho1 mutant (Helliwell et al. 1998b) suggested that the PKC1-activated MAP kinase pathway might be required for repolarization. To investigate this further, we examined repolarization of the actin cytoskeleton and FKS1 in bck1 and mpk1 null mutants. As described above, the actin cytoskeleton and FKS1 were normally depolarized in bck1 and mpk1 cells shifted to 37°C for 35 min (Fig. 4). However, after 120 min and longer, when the actin cytoskeleton is repolarized in wild-type cells, depolarization of the actin cytoskeleton persisted in most bck1 and mpk1 cells (Fig. 4). A similar effect was obtained when examining FKS1 distribution (data not shown). It is important to note that bck1 and mpk1 null mutations in our strain background do not confer a severe temperature sensitive growth defect, as reported for other strain backgrounds, and therefore the persistent depolarization of the actin cytoskeleton and FKS1 cannot be attributed to cell death. Thus, the PKC1-activated MAP kinase cascade is required for repolarization rather than depolarization of cell growth.
Figure 4.
BCK1 and MPK1 are required for repolarization of the actin cytoskeleton upon heat shock. Wild-type (A–F, JK9-3da), bck1 (G–L, PA109-1c), and mpk1 (M–R, TS45-1a) cells were grown in rich medium at 24°C and shifted to 37°C for 35 and 180 min. Samples were fixed, stained with TRITC-phalloidin, and observed by fluorescence (A, C, E, G, I, K, M, O, and Q) and Nomarski (B, D, F, H, J, L, N, P, and R) microscopy to visualize the actin cytoskeleton and whole cells, respectively.
Stress-induced Depolarization of WSC1 Distribution Is Independent of a Depolarized Actin Cytoskeleton yet WSC1 Localization Is Controlled by the Actin Cytoskeleton
To determine if cell wall stress affects localization of the plasma membrane protein WSC1, we constructed a COOH terminally, 3×HA-tagged, functional WSC1 (pWSC1-HA) (see Materials and Methods). Visualization of WSC1 by indirect immunofluorescence revealed a polarized distribution similar to that of FKS1 and other proteins found at a growth (bud) site (Fig. 5). Surprisingly, WSC1 had previously been reported to be evenly distributed throughout the cell periphery (Verna et al. 1997). This discrepancy may be because of the earlier study using overexpressed WSC1, whereas we examined WSC1 expressed from its own promoter on a centromeric plasmid. To examine WSC1 localization in response to stress, growing wild-type cells containing pWSC1-HA were shifted from 24 to 37°C, incubated at the higher temperature for 35 and 120 min, and processed for visualization of WSC1 (WSC1-HA). After 35 min, WSC1 was dispersed throughout the cell periphery (Fig. 5). After 120 min at 37°C, WSC1 was repolarized (data not shown). Thus, like the actin cytoskeleton and FKS1, WSC1 displayed a transient depolarization upon heat shock. Interestingly, WSC1 was also found in internal compartments at both low and high temperatures.
Figure 5.
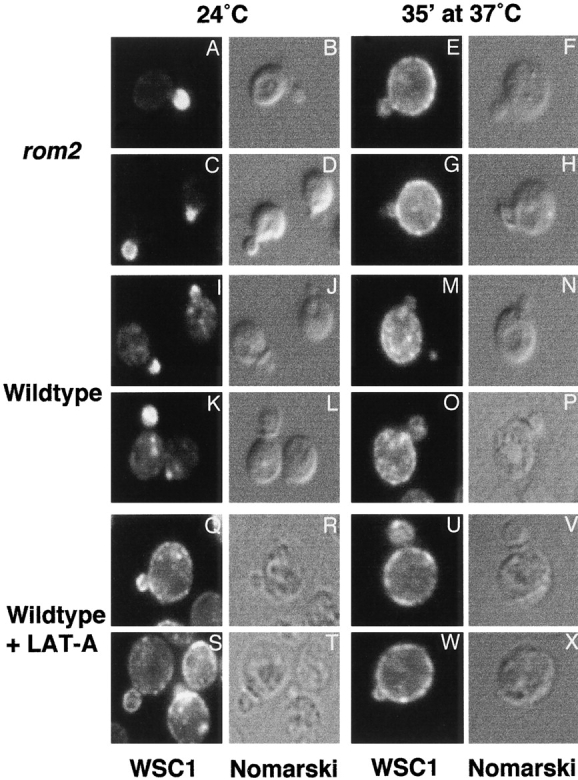
Stress-induced depolarization of WSC1 distribution is independent of a depolarized actin cytoskeleton, yet WSC1 localization is controlled by the actin cytoskeleton. Wild-type (I–X, JK9-3da) or rom2 (A–H, AS138-1b) cells expressing WSC1-HA (pWSC1-HA) were grown in minimal medium at 24°C, shifted to 37°C for 35 min or maintained at 24°C, fixed, and processed for detection of WSC1-HA by immunofluorescence (see Materials and Methods). In cells grown continuously at 24°C, LAT-A was added 35 min before fixation (Q–T). In cells shifted to 37°C, LAT-A was added at the time of shift (U–X). Fluorescence (A, C, E, G, I, K, M, O, Q, S, U, and W) and Nomarski (B, D, F, H, J, L, N, P, R, T, V, and X) images are shown.
We asked if, like for FKS1, stress-induced depolarization of WSC1 is LAT-A sensitive and, thus, dependent on the actin cytoskeleton. Interestingly, WSC1 was depolarized in LAT-A–treated cells grown at both low and high temperatures, indicating that LAT-A treatment induces WSC1 depolarization independently of stress (Fig. 5). Thus, LAT-A could not be used to determine if stress-induced WSC1 depolarization requires the actin cytoskeleton. However, this experiment demonstrates that WSC1 responds to a change in the actin cytoskeleton in addition to controlling the actin cytoskeleton, as shown above.
To determine if the stress-induced depolarization of WSC1 is dependent on a depolarized actin cytoskeleton, we investigated WSC1 distribution in heat shocked rom2 cells. As reported above, a rom2 mutation blocks stress-induced depolarization of the actin cytoskeleton and FKS1. WSC1 distribution was still depolarized in a rom2 mutant shifted to a high temperature for 35 min (Fig. 5). Thus, WSC1 depolarization is independent of depolarization of the actin cytoskeleton, suggesting that WSC1 may be a stress-specific actin landmark in addition to a signal transducer (Gray et al. 1997; Verna et al. 1997; Jacoby et al. 1998). The above results taken together suggest that WSC1, like mammalian integrins, both controls and responds to the actin cytoskeleton (see Discussion).
Discussion
We have shown that the actin cytoskeleton and the distribution of the cell wall synthesis enzyme glucan synthase (FKS1 and its regulatory subunit RHO1) are transiently depolarized in response to cell wall stress. In heat shocked or SDS-treated cells, actin patches and glucan synthase are redistributed from a polarized growth site (bud) to the periphery of the entire cell. This stress-induced depolarization of cell growth, possibly as a mechanism to repair general cell wall damage, is mediated by a putative signaling pathway consisting of the plasma membrane protein WSC1, the RHO1 exchange factor ROM2, RHO1, PKC1, and a yet-to-be identified PKC1 effector branch. The PKC1-activated MAP kinase pathway mediates repolarization of the actin cytoskeleton and FKS1. The de- and repolarization of FKS1 is dependent on the actin cytoskeleton. A model summarizing our results is shown in Fig. 6.
Figure 6.
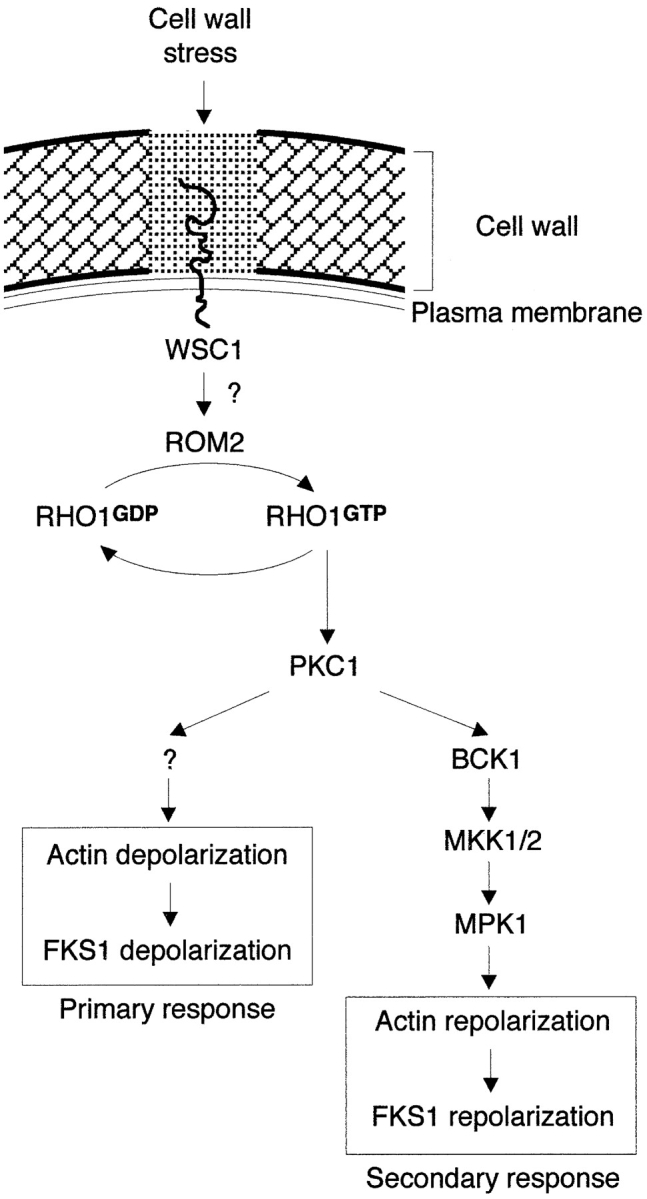
Model for the regulation of actin cytoskeleton and FKS1 distribution upon cell wall stress (see Discussion for further details).
The type I transmembrane protein WSC1 is possibly a signal transducer that senses and signals the integrity of the cell wall. A wsc1 mutant is defective in stress-induced depolarization and in activation of MPK1, sensitive to cell wall stress (SDS and heat), and is suppressed by overexpression of ROM2, RHO1, or PKC1 (Gray et al. 1997; Verna et al. 1997; Jacoby et al. 1998). The mechanisms by which WSC1 senses and signals cell wall integrity remain to be determined. WSC1 might sense and respond to a turgor pressure–induced outward stretching of the plasma membrane that results from a cell wall defect. Consistent with this possibility, several treatments that stretch the plasma membrane induce a depolarization response. Chlorpromazine, a cationic amphipath that inserts into and stretches the plasma membrane, induces depolarization of the actin cytoskeleton (Delley, P.-A., and M.N. Hall, unpublished results) and MPK1 activation (Kamada et al. 1995). Hyperosmotic shock also induces depolarization of the actin cytoskeleton (Chowdhury et al. 1992). Depolarization of the actin cytoskeleton in response to chlorpromazine or hyperosmotic shock is partly blocked in a wsc1 or rom2 mutant (Delley, P.-A., and M.N. Hall, unpublished results). Alternatively, WSC1 might interact with and directly sense the cell wall (Lodder et al. 1999). It also remains to be determined if WSC1 indeed controls ROM2 activity and, if so, how this might be achieved.
In addition to its role as a putative sensor and signal transducer, WSC1 might also serve as a stress-specific actin landmark. WSC1 also depolarizes upon heat shock and this depolarization is independent of a depolarization of the actin cytoskeleton. Interestingly, although WSC1 may control the actin cytoskeleton both as a signal transducer and a landmark, it also responds to the actin cytoskeleton as LAT-A–induced depolymerization of the actin cytoskeleton also causes WSC1 depolarization. Thus, there appears to be a bidirectional signaling between WSC1 and the actin cytoskeleton. A similar bidirectional signaling between integrins and the actin cytoskeleton has been observed in mammalian cells (Schoenwaelder and Burridge 1999).
The RHO1 GTPase switch and the RHO1 effector PKC1 mediate the transient depolarization response. ROM2 is necessary and hyperactivated RHO1 or PKC1 is sufficient to induce depolarization of the actin cytoskeleton and FKS1. Interestingly, RHO1 and PKC1 are also required for polarization of the actin cytoskeleton. First, loss-of-function rho1 mutants are defective in polarization of the actin cytoskeleton (Helliwell et al. 1998b). Second, overexpression of PKC1 restores actin organization in a rho1 mutant (Helliwell et al. 1998b). Third, overexpression of RHO1 or PKC1 rescues a tor2 mutant defective in polarized organization of the actin cytoskeleton (Schmidt et al. 1997; Helliwell et al. 1998a). The finding that activated RHO1 and its effector PKC1 control both polarization and depolarization of the actin cytoskeleton suggest that RHO1 may be a more finely-tuned switch than previously thought. RHO1 in a hyperactive state, as suggested by our findings, may cause depolarization, whereas RHO1 in a normally active state mediates polarization. Thus, RHO1 may function as a molecular rheostat, with more than one on position, rather than as a simple on-off switch. A second on position could require another RHO1 regulator in addition to ROM2. Alternatively, RHO1 may still function as a simple on-off switch, and depolarization may result from activation of a larger or a specific pool of RHO1.
PKC1, like RHO1, also appears to mediate both depolarization and repolarization of the actin cytoskeleton and FKS1. However, unlike RHO1, PKC1 may mediate these two different responses via different effectors. First, mutants defective in MPK1/SLT2 exhibit a depolarized actin cytoskeleton (Mazzoni et al. 1993). Second, overexpression of MPK1 suppresses the actin polarization defect of an rho1 mutant (Helliwell et al. 1998b). Third, bck1 and mpk1 mutants are not defective in stress-induced actin and FKS1 depolarization (Fig. 4). Fourth, BCK1 and MPK1 are necessary to restore polarization of the actin cytoskeleton and FKS1 after cell wall stress (Fig. 4). Furthermore, hyperactivated BCK1 or MKK1 does not induce actin and FKS1 depolarization (Fig. 3). These findings suggest that the PKC1-activated MAP kinase cascade mediates repolarization, and that a PKC1 effector branch other than this well characterized MAP kinase cascade mediates depolarization. Although the components of a second PKC1 effector branch have not been identified, the existence of a signaling bifurcation below PKC1 has been proposed previously (Lee and Levin 1992; Helliwell et al. 1998b).
RHO1 and PKC1 may control both depolarization and repolarization by activating the two PKC1 effector pathways at different times. Activation of the yet-to-be defined PKC1 effector branch, which causes depolarization (primary response), may occur rapidly and only upon stress-induced hyperactivation of RHO1. Activation of the PKC1-activated MAP kinase cascade may be a delayed response that results in repolarization (secondary response). Interestingly, Kamada et al. 1995 reported that the MPK1 MAP kinase is maximally activated after 30 min of heat shock, which is significantly delayed compared with the rapid induction (1–2 min) of MPK1 or the HOG1 MAP kinase by hypotonic or hypertonic stress, respectively (Chowdhury et al. 1992; Brewster et al. 1993). When we use the same regimen to heat shock cells as that used by Kamada et al. 1995, we find that the maximum percentage of depolarized cells is reached within ∼15 min (Delley, P.-A., and M.N. Hall, unpublished results), suggesting that the actin depolarization response precedes activation of MPK1.
The actin-dependent depolarization of glucan synthase (FKS1 and RHO1) in response to cell wall stress may be a homeostasis mechanism to repair general cell wall damage. Lillie and Brown 1994 reported that MYO2, a yeast class V myosin, and SMY1, a MYO2-interacting protein, disappear from and reappear at the bud upon heat shock, within a time course similar to that of actin de- and repolarization. MYO2 plays a role in the transport of secretory vesicles along actin cables to the growth site, and is, thus, involved in polarized growth and secretion. It will be of interest to determine if depolarization of the integral membrane protein FKS1 involves depolarized secretion of newly made FKS1 or dispersal of previously polarized FKS1. Attempts to address this issue by examining FKS1 localization in heat-stressed and cycloheximide-treated cells were inconclusive because cycloheximide alone affects actin organization. Furthermore, the mechanism by which the peripheral membrane protein RHO1 is redistributed may be different from that of FKS1. Unlike depolarization of FKS1, depolarization of RHO1 was not prevented by LAT-A treatment or by a rom2 or wsc1 mutation (Delley, P.-A., and M.N. Hall, unpublished results).
The regulation of FKS1 de- and repolarization by RHO1 is the third level on which RHO1 controls FKS1. In addition to controlling FKS1 localization, RHO1 also controls transcription of glucan synthase encoding genes (Igual et al. 1996; Zhao et al. 1998), and is a regulatory subunit of the glucan synthase complex (Drgonova et al. 1996; Qadota et al. 1996; Cabib et al. 1998).
Acknowledgments
We thank Thomas Beck, Sylvane Desrivières, Philipp Ernst, Joe Gray, Isabelle Howald, David Levin, Kunihiro Matsumoto, Tobias Schmelzle, and Anja Schmidt for plasmids and strains, and Yoshikazu Ohya for antibody.
Preparation of LAT-A was supported by the National Institutes of Health grant CA 47135 to Philipp Crews. This research was supported by grants from the Canton of Basel and the Swiss National Science Foundation awarded to M.N. Hall.
Footnotes
1.used in this paper: GTPase, guanosine triphosphatase; HA, hemagglutinin; LAT-A, latrunculin A; MAP, mitogen-activated protein
References
- Adams A.E., Pringle J.R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic mutant Saccharomyces cerevisiae . J. Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A.S., Bouquin N., Johnston L.H., Treisman R. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J. Biol. Chem. 1998;273:8616–8622. doi: 10.1074/jbc.273.15.8616. [DOI] [PubMed] [Google Scholar]
- Amberg D.C. Three-dimensional imaging of the yeast actin cytoskeleton through the budding cell cycle. Mol. Biol. Cell. 1998;9:3259–3262. doi: 10.1091/mbc.9.12.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F. Signalling in the yeastsan informational cascade with links to the filamentous fungi. Microbiol. Mol. Biol. Rev. 1998;62:249–274. doi: 10.1128/mmbr.62.2.249-274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti H., Raths S., Crausaz F., Riezman H. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol. Biol. Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickle M., Delley P.A., Schmidt A., Hall M.N. Cell wall integrity modulates RHO1 activity via the exchange factor ROM2. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2235–2245. doi: 10.1093/emboj/17.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster J.L., de Valoir T., Dwyer N.D., Winter E., Gustin M.C. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Brunelli J.P., Pall M.L. A series of yeast shuttle vectors for expression of cDNAs and other DNA sequences. Yeast. 1993;9:1299–1308. doi: 10.1002/yea.320091203. [DOI] [PubMed] [Google Scholar]
- Cabib E., Drgonova J., Drgon T. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem. 1998;67:307–333. doi: 10.1146/annurev.biochem.67.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J. Generation of cell polarity in yeast. Curr. Opin. Cell Biol. 1996;8:557–565. doi: 10.1016/s0955-0674(96)80035-4. [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Smith K.W., Gustin M.C. Osmotic stress and the yeast cytoskeletonphenotype-specific suppression of an actin mutation. J. Cell Biol. 1992;118:561–571. doi: 10.1083/jcb.118.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coue M., Brenner S.L., Spector I., Korn E.D. Inhibition of actin polymerization by latrunculin A. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Davenport K.R., Sohaskey M., Kamada Y., Levin D.E., Gustin M.C. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 1995;270:30157–30161. doi: 10.1074/jbc.270.50.30157. [DOI] [PubMed] [Google Scholar]
- Desrivières S., Cooke F.T., Parker P.J., Hall M.N. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae . J. Biol. Chem. 1998;273:15787–15793. doi: 10.1074/jbc.273.25.15787. [DOI] [PubMed] [Google Scholar]
- Drgonova J., Drgon T., Tanaka K., Kollar R., Chen G.C., Ford R.A., Chan C.S., Takai Y., Cabib E. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996;272:277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- Evangelista M., Blundell K., Longtine M.S., Chow C.J., Adames N., Pringle J.R., Peter M., Boone C. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- Gietz R.D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Gray J.V., Ogas J.P., Kamada Y., Stone M., Levin D.E., Herskowitz I. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:4924–4937. doi: 10.1093/emboj/16.16.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin M.C., Albertyn J., Alexander M., Davenport K. MAP kinase pathways in the yeast Saccharomyces cerevisiae . Microbiol. Mol. Biol. Rev. 1998;62:1264–1300. doi: 10.1128/mmbr.62.4.1264-1300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie J., Fink G.R. Guide to Yeast Genetics and Molecular Biology 1991. Academic Press; San Diego: pp. 933 [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Helliwell S.B., Howald I., Barbet N., Hall M.N. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae Genetics 148 1998. 99 112a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell S.B., Schmidt A., Ohya Y., Hall M.N. The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton Curr. Biol 8 1998. 1211 1214b [DOI] [PubMed] [Google Scholar]
- Howe A., Aplin A.E., Alahari S.K., Juliano R.L. Integrin signaling and cell growth control. Curr. Opin. Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Igual J.C., Johnson A.L., Johnston L.H. Coordinated regulation of gene expression by the cell cycle transcription factor Swi4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:5001–5013. [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby J.J., Nilius S.M., Heinisch J.J. A screen for upstream components of the yeast protein kinase C signal transduction pathway identifies the product of the SLG1 gene. Mol. Gen. Genet. 1998;258:148–155. doi: 10.1007/s004380050717. [DOI] [PubMed] [Google Scholar]
- Kamada Y., U.S. Jung, J. Piotrowski, and D.E. Levin The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Kamada Y., Qadota H., Python C.P., Anraku Y., Ohya Y., Levin D.E. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 1996;271:9193–9196. doi: 10.1074/jbc.271.16.9193. [DOI] [PubMed] [Google Scholar]
- Kapteyn J.C., Van Den Ende H., Klis F.M. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta. 1999;1426:373–383. doi: 10.1016/s0304-4165(98)00137-8. [DOI] [PubMed] [Google Scholar]
- Karpova T.S., McNally J.G., Moltz S.L., Cooper J.A. Assembly and function of the actin cytoskeleton of yeastrelationships between cables and patches. J. Cell Biol. 1998;142:1501–1517. doi: 10.1083/jcb.142.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketela T., Green R., Bussey H. Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the Pkc1-Mpk1 cell integrity pathway. J. Bacteriol. 1999;181:3330–3340. doi: 10.1128/jb.181.11.3330-3340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Kaneko T., Yamamoto Y. Lysis of viable yeast cells by enzymes of Arthrobacter luteus. II. Purification and properties of an enzyme, zymolyase, which lyses viable yeast cells. J. Gen. Appl. Microbiol. 1974;20:323–344. [Google Scholar]
- Kohno H., Tanaka K., Mino A., Umikawa M., Imamura H., Fujiwara T., Fujita Y., Hotta K., Qadota H., Watanabe T., Ohya Y., Takai Y. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6060–6068. [PMC free article] [PubMed] [Google Scholar]
- Kollar R., Reinhold B.B., Petrakova E., Yeh H.J., Ashwell G., Drgonova J., Kapteyn J.C., Klis F.M., Cabib E. Architecture of the yeast cell wall. Beta(1→6)-glucan interconnects mannoprotein, beta(1→3)-glucan, and chitin. J. Biol. Chem. 1997;272:17762–17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- Leberer E., Thomas D.Y., Whiteway M. Pheromone signalling and polarized morphogenesis in yeast. Curr. Opin. Genet. Dev. 1997;7:59–66. doi: 10.1016/s0959-437x(97)80110-4. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Levin D.E. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 1992;12:172–182. doi: 10.1128/mcb.12.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D.E., Bowers B., Chen C.Y., Kamada Y., Watanabe M. Dissecting the protein kinase C/MAP kinase signalling pathway of Saccharomyces cerevisiae . Cell. Mol. Biol. Res. 1994;40:229–239. [PubMed] [Google Scholar]
- Li R., Zheng Y., Drubin D.G. Regulation of cortical actin cytoskeleton assembly during polarized cell growth in budding yeast. J. Cell Biol. 1995;128:599–615. doi: 10.1083/jcb.128.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie S.H., Brown S.S. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae . J. Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder A.L., Lee T.K., Ballester R. Characterization of the Wsc1 protein, a putative receptor in the stress response of Saccharomyces cerevisiae . Genetics. 1999;152:1487–1499. doi: 10.1093/genetics/152.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden K., Snyder M. Cell polarity and morphogenesis in budding yeast. Annu. Rev. Microbiol. 1998;52:687–744. doi: 10.1146/annurev.micro.52.1.687. [DOI] [PubMed] [Google Scholar]
- Manning B.D., Padmanabha R., Snyder M. The Rho-GEF Rom2p localizes to sites of polarized growth and participates in cytoskeleton functions in Saccharomyces cerevisiae . Mol. Biol. Cell. 1997;8:1829–1844. doi: 10.1091/mbc.8.10.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni C., Zarov P., Rambourg A., Mann C. The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae . J. Cell Biol. 1993;123:1821–1833. doi: 10.1083/jcb.123.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J., Preuss D., Moon A., Wong A., Drubin D., Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J. Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka H., Tanaka K., Hirano H., Fujiwara T., Kohno H., Umikawa M., Mino A., Takai Y. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:5931–5938. doi: 10.1002/j.1460-2075.1995.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlean P. Biogenesis of yeast cell wall and surface components Pringle J., Broach J., Jones E. The Molecular and Cellular Biology of the Yeast Saccharomyces cerevisiaeCell Cycle and Cell Biology 1997. Cold; Spring Harbor Laboratory Press: 229–362.Cold Spring Harbor, NY [Google Scholar]
- Ozaki K., Tanaka K., Imamura H., Hihara T., Kameyama T., Nonaka H., Hirano H., Matsuura Y., Takai Y. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:2196–2207. [PMC free article] [PubMed] [Google Scholar]
- Qadota H., Python C.P., Inoue S.B., Arisawa M., Anraku Y., Zheng Y., Watanabe T., Levin D.E., Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- Rajavel M., Philip B., Buehrer B.M., Errede B., Levin D.E. Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae . Mol. Cell. Biol. 1999;19:3969–3976. doi: 10.1128/mcb.19.6.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read E.B., Okamura H.H., Drubin D.G. Actin- and tubulin-dependent functions during Saccharomyces cerevisiae mating projection formation. Mol. Biol. Cell. 1992;3:429–444. doi: 10.1091/mbc.3.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1989. [Google Scholar]
- Schmidt A., Hall M.N. Signaling to the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Bickle M., Beck T., Hall M.N. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell. 1997;88:531–542. doi: 10.1016/s0092-8674(00)81893-0. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S.M., Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr. Opin. Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Takai Y. Control of reorganization of the actin cytoskeleton by Rho family small GTP-binding proteins in yeast. Curr. Opin. Cell Biol. 1998;10:112–116. doi: 10.1016/s0955-0674(98)80093-8. [DOI] [PubMed] [Google Scholar]
- Verna J., Lodder A., Lee K., Vagts A., Ballester R. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. USA. 1997;94:13804–13809. doi: 10.1073/pnas.94.25.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A., Brachat A., Pohlmann R., Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae . Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Waddle J.A., Karpova T.S., Waterston R.H., Cooper J.A. Movement of cortical actin patches in yeast. J. Cell Biol. 1996;132:861–870. doi: 10.1083/jcb.132.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Irie K., Matsumoto K. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 1995;15:5740–5749. doi: 10.1128/mcb.15.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamochi W., Tanaka K., Nonaka H., Maeda A., Musha T., Takai Y. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae . J. Cell Biol. 1994;125:1077–1093. doi: 10.1083/jcb.125.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzov P., Mazzoni C., Mann C. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:83–91. [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Jung U.S., Garrett-Engele P., Roe T., Cyert M.S., Levin D.E. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 1998;18:1013–1022. doi: 10.1128/mcb.18.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]