Abstract
The distribution of brain-derived neurotrophic factor (BDNF) in the song system of male zebra finches changes with posthatching age. At day 20, the hyperstriatum ventrale, pars caudale is the only song nucleus in which neurons showed BDNF immunoreactivity. At day 45, the staining in hyperstriatum ventrale, pars caudale was denser than at day 20 and the robust nucleus of the archistriatum, another song nucleus, showed BDNF labeling. By day 65, two additional song nuclei, area X and the lateral magnocellular nucleus of the anterior neostriatum, have become immunoreactive. In the adult, however, the amount of BDNF labeling in all of these brain nuclei is sharply reduced. These sequential events, the anatomical connections between these song nuclei, and the labeling of relevant axons and terminals suggest anterograde transport of BDNF. Furthermore, the timing of BDNF expression coincident with the development of singing behavior suggests that this neurotrophin may be directly involved with the differentiation of the song system.
Neurotrophins such as the brain-derived neurotrophic factor (BDNF) regulate the survival and differentiation of neuronal populations during early development, and play a significant role in the maintenance of established neurons or circuits in adults (1–8). Aside from the well-established retrograde action of BDNF (9, 10), recent evidence also shows that this neurotrophin can be anterogradely transported and could have permanent transynaptic effects (11). We used a highly specific antibody against the BDNF protein to investigate the distribution of this neurotrophin within the developing song control system of the male zebra finch (12) (Poephila guttata). This system provides a good model for studying the role of BDNF in the differentiation of neural circuits that control a specific behavior, because the song system’s discrete nuclei and the connections between them develop relatively late in posthatching life (13–15). We find that BDNF immunoreactivity appears sequentially in different song nuclei, suggesting anterograde transport of this protein. The neurotrophin also occurs primarily during a restricted period in which both the neural substrates for song and the singing behavior develop.
MATERIALS AND METHODS
Animals.
Experimental subjects were male zebra finches (P. guttata) that were obtained from a breeding colony in our animal facilities. This colony provided us with both adult males (n = 5; >90 days old) and young birds of known posthatching ages: 20 days old (n = 5), 45 days old (n = 4), and 65 days old (n = 6).
BDNF Antibody and Tissue Preparation.
The BDNF antibody was a gift from Amgen and is characterized as a polyclonal, affinity purified rabbit-anti-BDNF IgG antibody that has shown little or no crossreactivity to nerve growth factor, neurotrophin 3, or neurotrophin 4/5 (16, 17). Our preliminary tests confirmed earlier reports (17) that binding of the BDNF antibody to neural tissue is easily suppressed by overfixation. We therefore utilized a “limited fixation” protocol throughout this study. In this method, birds were first overdosed with Equithesin then transcardially perfused with 0.9% saline followed by perfusion with ≈200 ml of 2% paraformaldehyde in 25 mM phosphate buffer (PB, pH 7.4) for 30 min. The fixative was flushed out of the tissue by further perfusion with ≈250 ml of 10% sucrose in PB for an additional 45 min. The brain was immediately removed, the dura mater taken off, and the brain cryoprotected overnight in 30% sucrose in PB (4°C). The next day, the brains were cut in the sagittal plane at 30 μm on a freezing microtome and collected in cold PB.
Immunohistochemistry.
Free-floating sections were processed for immunohistochemistry by first washing every third section in two changes of PB over 30 min (room temperature). The sections were then blocked for 1 hr in 5% normal rabbit serum (GIBCO), 0.1% BSA (Sigma), and 0.1% Triton X-100 (Sigma) in PB. Sections were incubated for 48 hr (4°C) with either the BDNF antibody or a control rabbit IgG (Zymed) at a concentration of 0.3 μg/ml. After extensive washing, a secondary biotinylated goat anti-rabbit antibody was used at a concentration of 1.5 μg/ml., then washed and detected by using the avidin-biotin-peroxidase complex (Vector Elite ABC kit, Vector Laboratories) combined with nickel intensification (18). The sections were subsequently washed for an additional hour in PB, mounted onto subbed slides, cleared in xylene, and coverslipped with Permount (Fisher Scientific). Neurons were discriminated from glial cells by the presence of a large pale nucleus containing one or two distinct nucleoli. Alternate sections were counter-stained with cresyl-violet (Sigma) to verify the locations and boundaries of all of the brain areas examined in this study.
BDNF Optical Density Measurements.
Quantifications of the relative levels of immunoreactivity for BDNF in various parts of the song system were performed by using a modification of the image analysis method (19). Files of representative antibody processed sections were produced with a microscope-mounted digital camera. The images were analyzed for relative optical densities by the National Institutes of Health’s image program (20). Although all members of a given age group showed the same pattern of BDNF immunostaining, there were some individual variations in staining intensities caused by such factors as the level of fixation, binding sensitivity to the antibody, washing steps, and differences in the optimal development times to visualize the peroxidase substrate. To compensate for these differences, several random optical density measurements from several representative sections containing the song system area of interest were determined and averaged for each bird (mean value ± SD). This process was repeated on the same respective sections, but this time the optical density measurements were made in the areas immediately around and outside of the corresponding song nuclei. The mean optical density of the surrounding area was then subtracted from the mean song system optical density to give a measurement of the relative amounts of BDNF immunoreactivity for a given song nucleus. This subtracted mean optical density (SMOD) was determined for all birds using the same calibration scale. The error bars were generated from the SDs of the mean optical density values within or just outside of the particular song nucleus by taking the square root of the sum of the squared SDs.
RESULTS
BDNF Is Present in the Song System of Young Birds.
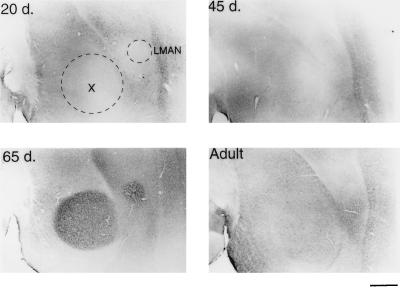
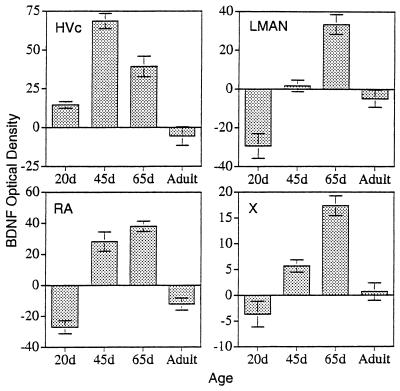
BDNF immunoreactivity was widespread throughout many different areas of the 20-day-old male zebra finch brain (n = 5). We will, however, mainly address the changes in the staining within the vocal control areas, or song system (Fig. 1). The telencephalic song system area, the hyperstriatum ventrale, pars caudale (HVc or high vocal center), stood out in both the number and density of labeled cells in contrast to its immediate vicinity (Figs. 2 and 3). Optical density quantifications of HVc in 20-day-old males, show a SMOD of 14.6 ± 2.2 (SMOD measurements are in arbitrary units). The labeled neurons appeared to be evenly distributed throughout the nucleus, whereas examination of individually labeled cells showed that the immunoreaction product was largely confined to the cytoplasm and fine neuronal processes, but very little staining, if any, occurred in the cell nucleus (Fig. 5). At this age, the robust nucleus of the archistriatum (RA) showed no BDNF immunostaining (Fig. 2). The SMOD of a 20-day-old male RA showed a negative value (relative to the surrounding archistriatum) of −27.0 ± 4.2. However, there were several labeled cells and fibers located between the dorsal-caudal boundaries just outside of RA and ventral to and including the posterior borders of the lamina archistriatalus dorsalis. This area of BDNF staining was previously reported in 20-day-old finches (21). Two other song system areas in the anterior telencephalon, area X in the lobus parolfactorius and the lateral magnocellular nucleus of the anterior neostriatum (LMAN) both showed no immunoreactivity (Fig. 4). The SMOD of area X was −3.65 ± 2.5, whereas the same measurement in LMAN was −29.4 ± 6.4. Contrary to a report by Johnson et al. (21), we did not detect labeled cells in LMAN. Similarly, the dorsolateral nucleus of the lateral thalamus (DLM) and the hypoglossal nucleus (nXIIts) that innervates the syrinx both showed very little, if any, BDNF staining (data not shown).
Figure 1.
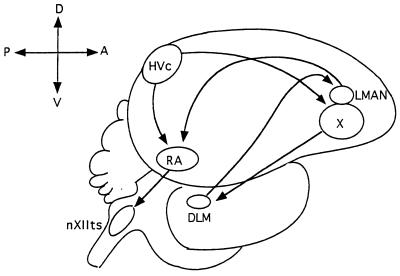
Schematic diagram of the adult male zebra finch song system. The HVc projects to both RA and area X. Area X sends its afferents to DLM, which connects to LMAN. The LMAN makes afferent connections to RA, which sends some of its descending projections to the nXIIts that innervates the syringeal muscles used in singing. Anterior is to the right, dorsal side is up.
Figure 2.
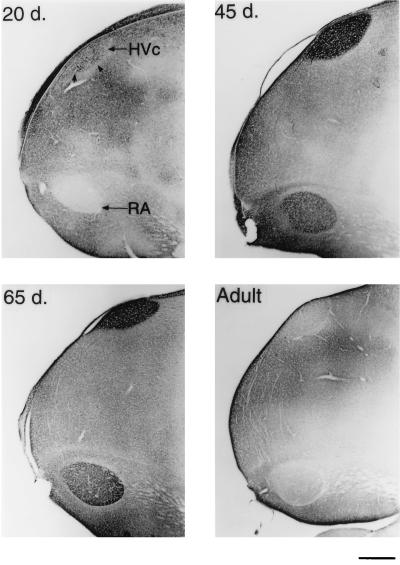
Sagittal sections of the caudal telencephalon stained for BDNF. A 20-day-old male has many BDNF containing cells, but little staining is seen in RA (Upper Left). By 45 days, BDNF staining is pronounced in HVc cells and neuropil, some of the axon bundles that connect HVc and RA, and in the RA neuropil only (Upper Right). In a 65-day-old male, BDNF staining within HVc is still heavy, but is mostly confined to the neuropil. The RA is also heavily labeled at this age (Lower Left). In the adult, however, BDNF immunostaining within these song nuclei is sharply reduced (Lower Right). (Bar = 500 μm.)
Figure 3.
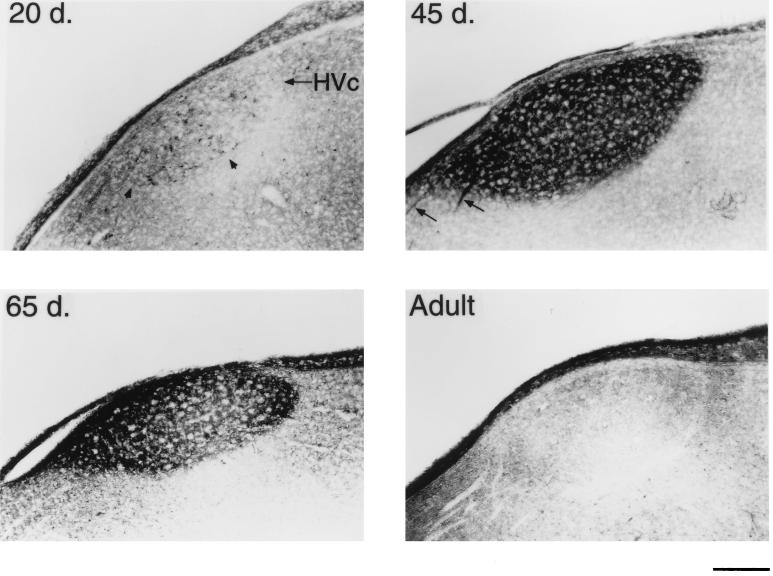
BDNF immunostaining of the male zebra finch HVc at different ages. A 20-day-old male already has many BDNF-containing neurons within HVc (Upper Left). At 45 days, staining specifically within HVc is very pronounced to both the cells and neuropil (Upper Right). Also seen at this age are several stained axon bundles that connect HVc to RA (arrows). A 65-day-old bird still shows heavy BDNF staining, but the labeling is largely confined to the neuropil (Lower Left). An adult bird shows little BDNF labeling (Lower Right). (Bar = 200 μm.)
Figure 5.
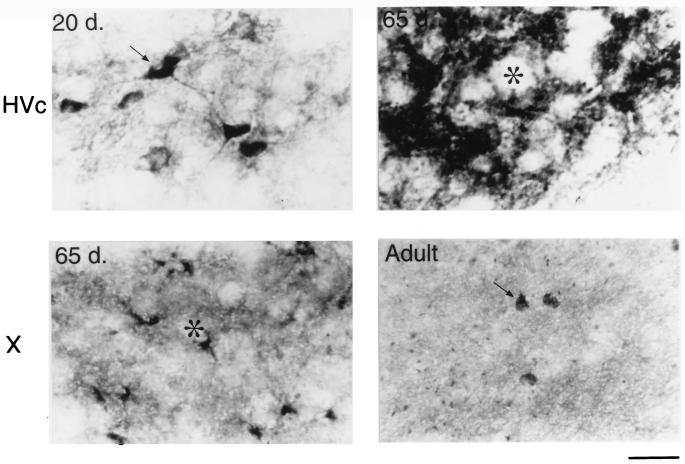
The different immunostaining patterns within HVc and area X of the developing male zebra finch. At 20 days of age, HVc contains many BDNF stained neurons and fine processes (Upper Left). At 65 days, however, only a few HVc cell bodies are labeled, but most of the staining shifts to the neuropil and/or extracellular matrix surrounding the unstained cells (asterisk, Upper Right). Also at 65 days, BDNF labeling in area X is rapidly increased and appears clustered around unlabeled cell bodies (asterisk, Lower Left). In the adult, however, there are many widely spaced neurons in area X that contain BDNF surrounded by many small knob-like objects (Lower Right). (Bar = 20 μm.)
Figure 4.
BDNF immunostaining of LMAN and area X in a male zebra finch at different ages. A 20-day-old male shows little BDNF staining in either LMAN or area X (dotted outlines, Upper Left). At 45 days (Upper Right), the level of immunostaining within these areas is still very low, but is rapidly and specifically increased in the 65-day-old bird (Lower Left). The BDNF staining is much lower in the adult LMAN and area X (Lower Right), when compared with the 65-day-old male. Anterior is to the right, dorsal side is up. (Bar = 500 μm.)
Juvenile Birds Exhibit a Dramatic Shift in BDNF Expression within the Song System.
Compared to 20-day-old males, in 45-day-old zebra finch males (n = 4), the BDNF labeling within HVc became noticeably darker. This sharp increase in BDNF staining was caused, in large part, by the extensive labeling now seen in the neuropil and/or extracellular matrix, but still specifically within the boundaries of HVc (Figs. 2 and 3). The SMOD of a male HVc at this age was 68.6 ± 4.9, which reflects an almost 5-fold increase in BDNF staining over the same brain area in a 20-day-old male (Fig. 6). Many immunoreactive cells were still evident in HVc. However, an important change that was observed during this age, was the occurrence now of several darkly stained axon bundles that could clearly be seen leaving HVc and going down to and entering RA (Fig. 3). At this age, RA showed moderate BDNF labeling, although none of the staining was cellular but accumulated outside of the unstained cell bodies. Nevertheless, the RA labeling was highly localized within the nuclear boundaries and the darkened RA could be clearly demarcated from the rest of the lightly stained archistriatum (Fig. 2). The SMOD of a 45-day-old male RA was 28.2 ± 6.2. Area X and LMAN still showed very little BDNF immunostaining in 45-day-old males with an SMOD of 5.7 ± 1.2 and 1.7 ± 3.0, respectively (Fig. 4). Both DLM and nXIIts also continued to show very little antibody staining.
Figure 6.
Quantification of the SMOD of BDNF in various song system nuclei as a function of age. In HVc, the moderate SMOD of a 20-day-old male is attributable to the many stained cells within the nucleus (Upper Left). The SMOD peaks in HVc at around 45 days of age. The other song system areas of RA, LMAN, and area X show peak levels of BDNF SMOD at a much later time of ≈65 days of age. The SMOD values for all of these song system areas drops sharply in the adult male (Lower Right).
In 65-day-old males (n = 6), few BDNF immunoreactive cells could be detected within HVc. The nucleus is still darkly stained in the neuropil, however, and this is reflected in the SMOD of 39.3 ± 6.6. Also during this age, the labeling of the axon bundles between HVc and RA is rarely observed. In the archistriatum, RA was strongly labeled with an SMOD of 38 ± 3.4, whereas only a few BDNF stained cells or fibers could be seen just outside of the dorsal-caudal RA area. The most striking change in BDNF labeling during this period occurred in area X and LMAN. Whereas previous age groups showed little staining within these two nuclei, at 65 days the male brain suddenly showed a sharp increase in BDNF labeling (Fig. 4). In area X, the SMOD was 17.85 ± 1.9, and in LMAN the SMOD was 33.4 ± 5.1. All of the new labeling in these areas was in the extracellular matrix. Under high magnification, the BDNF immunoreaction product in area X appeared punctate and clustered around unlabeled cell bodies (Fig. 5).
The Adult Song System Shows Little BDNF.
The song system of adult birds (4 months to >1 year old) showed conspicuously low levels of BDNF immunostaining regardless of whether the birds were quiescent (n = 2) or just sang (n = 3). In many cases, the levels of immunoreactivity in HVc, RA, and LMAN were lower than the staining of the immediate surrounding areas (Figs. 2–5). In HVc, for example, the SMOD of the adult was −5.5 ± 6.0, whereas in RA, the SMOD was −12.2 ± 3.9. Similarly, in LMAN, the SMOD dropped from the 65-day-old values to −4.9 ± 4.5. In area X, however, a new staining pattern emerged in the adult where many widely spaced cells were now labeled with the antibody. Surrounding the cells and mostly within the boundaries of area X were also many small knob-like immunoreactive objects (Fig. 5). However, because of their sparse distribution, the overall SMOD of the adult area X was only 0.7 ± 1.7. Staining in DLM and nXIIts continued to be very low and unchanged in the adult.
Controls Show Specificity of the BDNF Antibody.
The BDNF antibody staining specificity was verified in all age groups by either omitting the primary antibody or replacing it with a control rabbit-IgG. In either case, no immunoreaction product specifically accumulated in any brain area, including the song system. Also, as an internal control, the optical density of BDNF immunoreactivity in DLM was measured in 20-, 45-, 65-day-old, and adult birds with respective SMOD values of 0.36 ± 1.8, 0.4 ± 1.4, 0.2 ± 1.9, and 0.4 ± 2.3. These results show that the BDNF antibody did not bind specifically to DLM as a function of age, when this was true in other song system nuclei.
DISCUSSION
The results presented in this paper show that BDNF is transiently expressed in the song system of the male zebra finch during the period of early vocal development. Furthermore, the pattern of antibody labeling shows a spatial-temporal distribution that is suggestive of localized production of the trophic factor in HVc, followed by its transport later to other song system nuclei.
BDNF has diverse effects on both the development and maintenance of specific populations of neurons. Its modulatory functions include synapse elimination or pruning (22–26), sprouting (27, 28), modification of the efficacy of developing synapses (29–31), and activity-dependent synaptic strengthening mediated by CREB (32, 33). BDNF can also regulate the formation and development of entire neural circuits (25, 34–36). This function of the neurotrophin could involve anterograde transport and transynaptic action (11). Anterograde transport of BDNF is well documented in several systems including the medial habenula and central nucleus of the amygdyla in rats (16), the adult rodent striatum (37), the developing retinotectal projection of chicken embryos (11), and noradrenergic neurons in mice (19).
Many BDNF containing neurons are present in the HVc of a 20-day-old male before any immunoreactivity can be detected in any other parts of the song system, including both its afferent and efferent nuclei. Since the labeled cells are only seen in this song nucleus, HVc may be the sole source of BDNF production within the song system during early development. Of the four different age classes examined, the level of BDNF staining in HVc peaks at or around 45 days of age, whereas the highest levels of the neurotrophin in RA, area X, and LMAN occur later, at 65 days. This time lag and the earliest appearance of labeled neurons only in HVc suggest that BDNF is first produced and accumulated in this nucleus before it is exported to RA and area X, which are the afferent targets of HVc (38, 39). The most compelling direct evidence of anterograde transport of BDNF from HVc to RA, however, is provided by the heavily labeled axon bundles (in 45-day-old males) that connect the two nuclei. This fiber tract is dense and can be easily distinguished (Fig. 3). In contrast, the fibers from HVc to area X are diffuse and spread over a relatively wide area of the telencephalon. This sparse distribution makes the labeled axons from HVc to area X difficult to discern. However, the punctate labeling around the unstained area X cells in the 65-day-old bird (Fig. 5) is characteristic of labeled presynaptic terminals containing the anterogradely transported BDNF (16, 19). Retrograde transport of BDNF may also occur within the song system. Because HVc does not directly project to LMAN, the accumulation of BDNF in this nucleus at day 65 may be due to retrograde uptake from RA, through the LMAN collaterals that extend into area X (40–42), or both. Because DLM shows little BDNF staining at any age, it can be excluded as a possible source of BDNF for area X, leaving HVc as the only probable source of the neurotrophin.
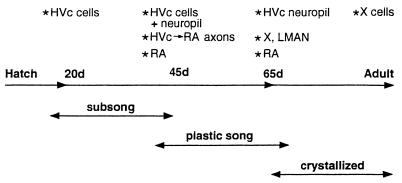
The functional significance of this brief and sequential expression of BDNF within the song system may be clarified by examination of the concomitant changes in vocal behavior. Zebra finches develop their final song in three phases, although the onset and length of each phase are not always age dependent but vary with auditory and other experiences (43, 44) (Fig. 7). The first is the subsong phase in which young birds of 20–40 days of age produce a rambling series of sounds that vary from delivery to delivery. The second is the plastic song phase in which young birds of 40–65 days of age produce songs whose syllables are somewhat variable. The third is the crystallization phase in which birds of 65–90 days of age complete song development by producing a stereotyped song pattern (45). It is during the plastic song phase when BDNF is most highly expressed within the song system. Furthermore, we find more specific correlations between some aspects of the timing and distribution of BDNF expression and singing behavior. Axons of HVc neurons wait for a while outside RA before making their afferent connections to the target neurons in RA at about 35 days of age (13). The timing of RA innervation corresponds to both the early stage of plastic song and the first appearance of BDNF in RA. Thereafter, the completion of innervation and synaptic rearrangement in RA continue until ≈day 65. Quantitative electron microscope studies in juvenile male zebra finches show a major increase and rearrangement of synaptic contacts within RA between 45 and 65 days of age, and were mainly attributed to the synapses originating from HVc neurons (46). The continued labeling of these two nuclei from day 45 to day 65 may reflect the role of BDNF in these processes. However, because the adult lacks BDNF staining in HVc and RA, the neurotrophin’s major role there is not likely to be in maintaining established cells or circuits. The volume of RA normally increases at or around 35 days of age, because both neuron size and the interneuronal spacing grow. Lesions of HVc in a 20 day-old male prevent the growth of size and spacing of RA neurons without affecting cell number (47). Thus, the primary effects of BDNF in RA may be not on cell survival but on these other aspects of differentiation.
Figure 7.
Representation of an idealized timeline correlating the posthatching age with the relative stages of vocal development and the corresponding changes in BDNF expression in the male zebra finch song system.
However, HVc’s influence may be more widespread and may affect the development of other song system nuclei as well. The acquisition of normal song requires the pathway from HVc to area X to DLM to LMAN to RA. Lesions of LMAN or area X in male zebra finches younger than 65 days of age cause abnormal song development (48–50). The abundance of BDNF in these two nuclei at day 65 may be related to the process that requires this pathway for proper song development. We know neither the source nor the significance of the labeled cells seen in the adult X area.
In conclusion, the first appearance of BDNF reflects the importance of HVc for the differentiation of the rest of the forebrain song system. The transport of BDNF to other song nuclei at the later stages of song development is consistent with the presumed roles played by these nuclei in song development. We therefore hypothesize that BDNF mediates the differentiation of the zebra finch’s song system to organize its neural circuits for the different stages of song development.
Acknowledgments
We are grateful to Dr. Q. Yan of Amgen for his generous gift of the BDNF polyclonal antibody. We also thank Paul Patterson for his critical reading of the manuscript.
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- HVc
hyperstriatum ventrale, pars caudale (or high vocal center)
- RA
robust nucleus of the archistriatum
- SMOD
subtracted mean optical density
- DLM
medial portion of the dorsolateral nucleus of the thalamus
- LMAN
lateral magnocellular nucleus of the anterior neostriatum
- nXIIts
hypoglossal nucleus
References
- 1.Lindsay R M, Thoenen H, Barde Y A. Dev Biol. 1985;112:319–328. doi: 10.1016/0012-1606(85)90402-6. [DOI] [PubMed] [Google Scholar]
- 2.Alderson R F, Alterman A L, Barde Y A, Lindsay R M. Neuron. 1990;5:297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- 3.Knusel B, Winslow J W, Rosenthal A, Burton L E, Seid D P, Nikulies K, Hefti F. Proc Natl Acad Sci USA. 1991;88:961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snider W D. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh A, Greenberg M E. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 6.Mamounas L A, Blue M E, Siuciak J A, Altar C A. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewin G R, Barde Y A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz P M, Borghesani P R, Levy R L, Pomeroy S L, Segal R A. Neuron. 1997;19:269–281. doi: 10.1016/s0896-6273(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 9.Levi-Montalcini R. Science. 1987;127:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 10.Oppenheim R W. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 11.von Bartheld C S, Byers M R, Williams R, Bothwell M. Nature (London) 1996;379:830–833. doi: 10.1038/379830a0. [DOI] [PubMed] [Google Scholar]
- 12.Nottebohm F, Stokes T M, Leonard C M. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 13.Konishi M, Akutagawa E. Nature (London) 1985;315:145–147. doi: 10.1038/315145a0. [DOI] [PubMed] [Google Scholar]
- 14.Bottjer S W, Miesner E A, Arnold A. Neurosci Lett. 1986;67:263–268. doi: 10.1016/0304-3940(86)90319-8. [DOI] [PubMed] [Google Scholar]
- 15.Kirn J R, DeVoogd T J. J Neurosci. 1989;9:3176–3187. doi: 10.1523/JNEUROSCI.09-09-03176.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conner J M, Lauterborn J C, Yan Q, Gell C M, Varon S. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan Q, Rosenfeld R D, Mathewon C R, Hawkins N, Lopez O T, Bennett L, Welcher A A. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- 18.Adams J C. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- 19.Fawcett J P, Bamji S X, Causing C G, Aloyz R, Ase A R, Reader T A, McLean J H, Miller F D. J Neurosci. 1998;18:2808–2821. doi: 10.1523/JNEUROSCI.18-08-02808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russ J C. The Image Processing Handbook. 2nd Ed. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- 21.Johnson F, Hohmann S E, DiStefano P S, Bottjer S W. J Neurosci. 1997;17:2101–2111. doi: 10.1523/JNEUROSCI.17-06-02101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Changeaux J P, Danchin A. Nature (London) 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 23.Purves D. Body and Brain: A Trophic Theory of Neural Connections. Cambridge, MA: Harvard Univ. Press; 1988. [DOI] [PubMed] [Google Scholar]
- 24.Vaughn J E. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- 25.Cabelli R J, Hohn A, Shatz C J. Science. 1995;2674:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 26.Henderson C E. Curr Opin Neurobiol. 1996;6:64–70. doi: 10.1016/s0959-4388(96)80010-9. [DOI] [PubMed] [Google Scholar]
- 27.Lo D C. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 28.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 29.Kang H, Schuman E M. Science. 1995;267:1652–1658. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 30.Figurov A, Pozzo-Miller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 31.Akeneya Y, Tsumoto T, Kinoshita S, Hatanaka H. J Neurosci. 1997;7:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao X, Finkbeiner S, Arnold D B, Shaywitz A J, Greenberg M E. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 33.Shieh P B, Hu S, Bobb K, Timmusk T, Ghosh A. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 34.Cohen-Corey S, Escandon E, Fraser S E. Dev Biol. 1996;179:102–115. doi: 10.1006/dbio.1996.0244. [DOI] [PubMed] [Google Scholar]
- 35.McAllister A K, Lo D C, Katz L C. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 36.McAllister A K, Katz L C, Lo D C. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 37.Altar C A, Cai N, Bliven T, Juhasz M, Conner J M, Acheson A L, Lindsay R M, Wiegand S J. Nature (London) 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 38.Katz L C, Gurney M E. Brain Res. 1981;211:192–197. doi: 10.1016/0006-8993(81)91073-8. [DOI] [PubMed] [Google Scholar]
- 39.Bottjer S W, Halsema K A, Brown S A, Miesner E A. J Comp Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- 40.Vates G E, Nottebohm F. Proc Natl Acad Sci USA. 1995;92:5139–5143. doi: 10.1073/pnas.92.11.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nixdorf-Bergweiler B E, Lips M B, Heinemann U. NeuroReport. 1995;6:1729–1732. doi: 10.1097/00001756-199509000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Livingstone F S, Mooney R. J Neurosci. 1997;17:8997–9009. doi: 10.1523/JNEUROSCI.17-23-08997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marler P. J Comp Physiol Psychol. 1970;71:1–25. [Google Scholar]
- 44.Slater P J B, Eales L A, Clayton N S. Adv Study Behav. 1988;18:1–33. [Google Scholar]
- 45.Immelmann K. In: Bird Vocalizations. Hinde R A, editor. London: Cambridge Univ. Press; 1969. pp. 61–74. [Google Scholar]
- 46.Herrmann K, Arnold A P. J Neurosci. 1991;11:2063–2074. doi: 10.1523/JNEUROSCI.11-07-02063.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akutagawa E, Konishi M. Proc Natl Acad Sci USA. 1994;91:12413–12417. doi: 10.1073/pnas.91.26.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bottjer S W, Miesner E A, Arnold A P. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 49.Sohrabji F, Nordeen E J, Nordeen K W. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 50.Scharff C, Nottebohm F. J Neurosci. 1991;11:2846–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]