Abstract
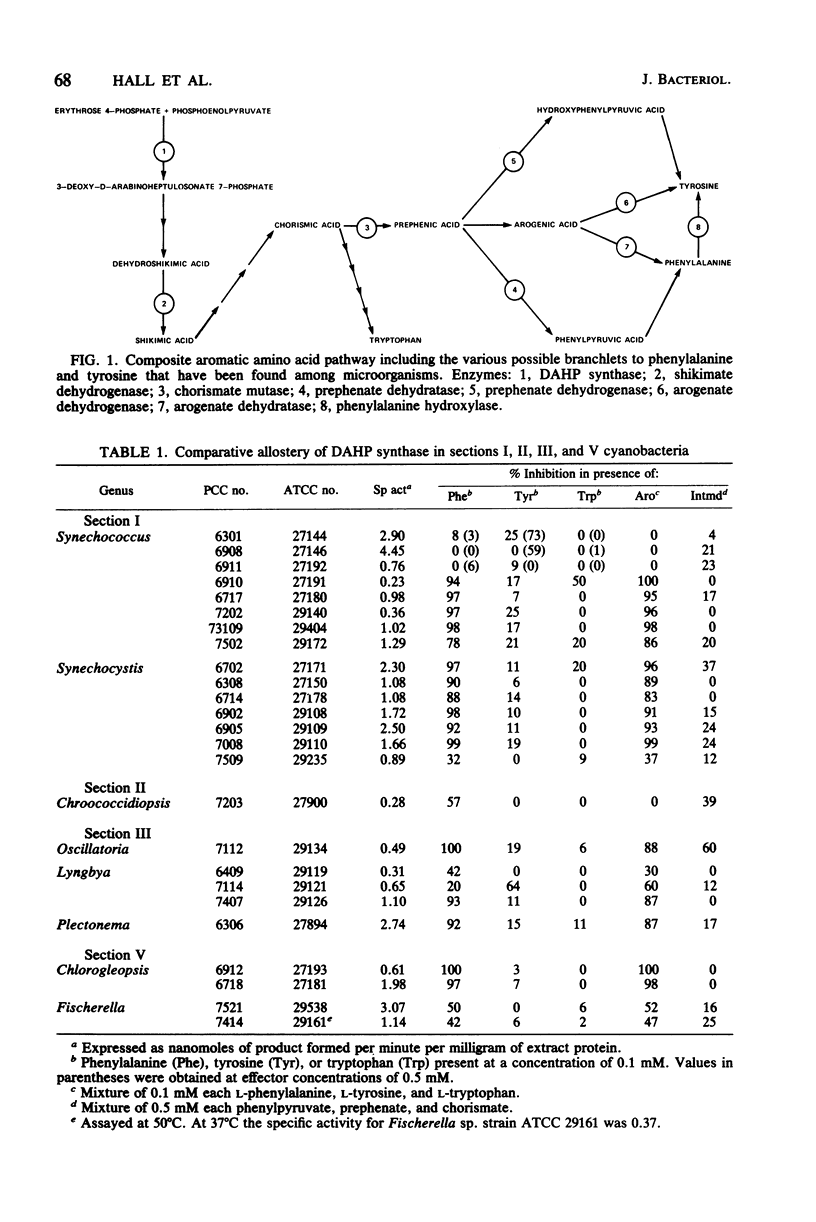
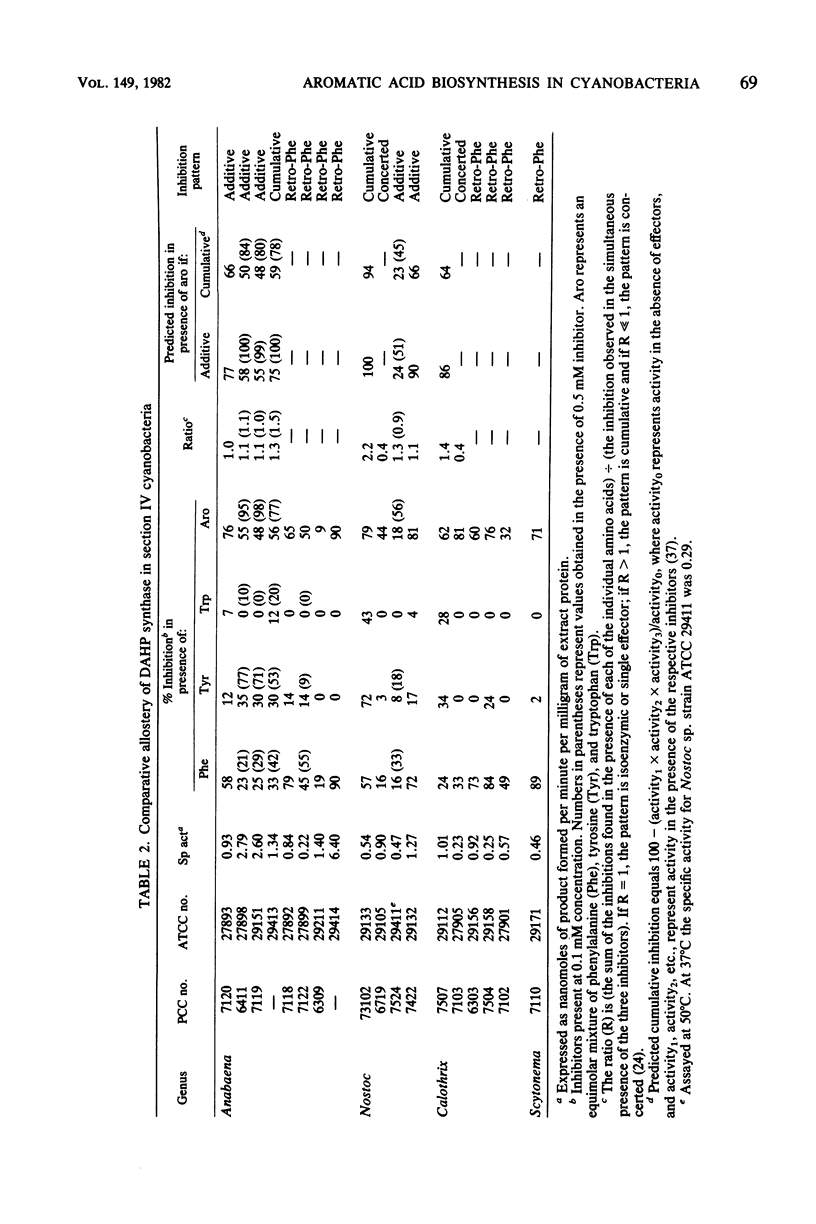
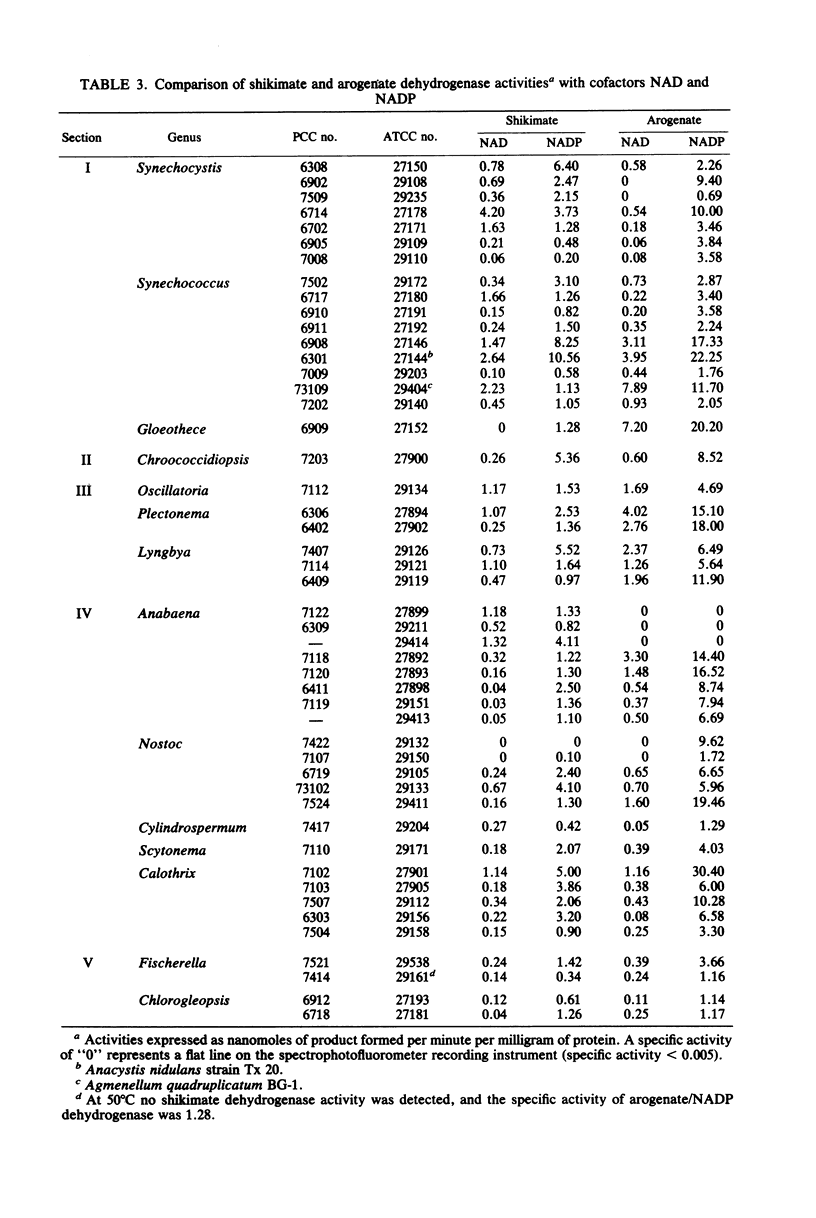
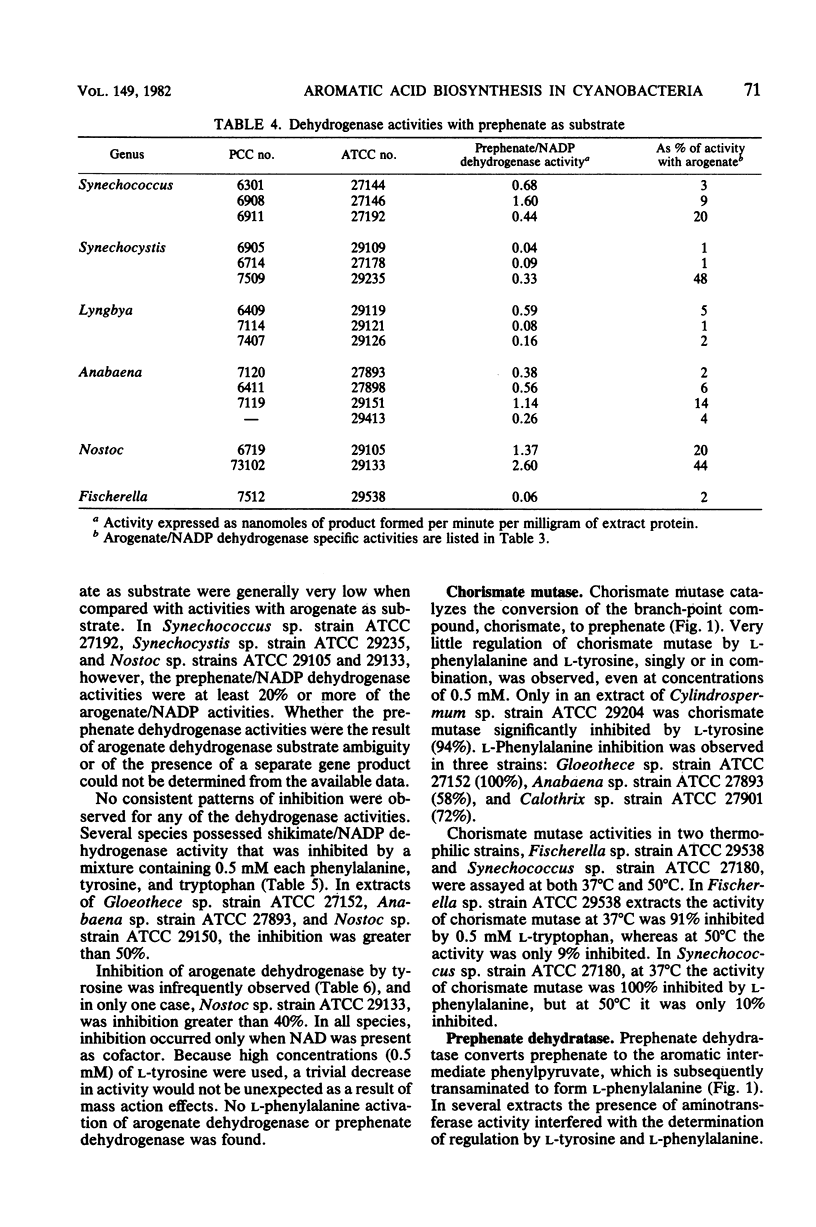
We examined the enzymology and regulatory patterns of the aromatic amino acid pathway in 48 strains of cyanobacteria including representatives from each of the five major grouping. Extensive diversity was found in allosteric inhibition patterns of 3-deoxy-D-arabinoheptulosonate 7-phosphate synthase, not only between the major groupings but also within several of the generic groupings. Unimetabolite inhibition by phenylalanine occurred in approximately half of the strains examined; in the other strains unimetabolite inhibition by tyrosine and cumulative, concerted, and additive patterns were found. The additive patterns suggest the presence of regulatory isozymes. Even though both arogenate and prephenate dehydrogenase activities were found in some strains, it seems clear that the arogenate pathway to tyrosine is a common trait that has been highly conserved among cyanobacteria. No arogenate dehydratase activities were found. In general, prephenate dehydratase activities were activated by tyrosine and inhibited by phenylalanine. Chorismate mutase, arogenate dehydrogenase, and shikimate dehydrogenase were nearly always unregulated. Most strains preferred NADP as the cofactor for the dehydrogenase activities. The diversity in the allosteric inhibition patterns for 3-deoxy-D-arabinoheptulosonate 7-phosphate synthase, cofactor specificities, and the presence or absence of prephenate dehydrogenase activity allowed the separation of subgroupings within several of the form genera, namely, Synechococcus, Synechocystis, Anabaena, Nostoc, and Calothrix.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode R., Birnbaum D. Die Enzyme der Biosynthese aromatischer Aminosäuren bei Hansenula henricii: Nachweis und Charakterisierung der Enzyme des Pretyrosin-Weges. Z Allg Mikrobiol. 1979;19(2):83–88. doi: 10.1002/jobm.3630190203. [DOI] [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F., Fox G. E. Cyanobacterial evolution: results of 16S ribosomal ribonucleic acid sequence analyses. Can J Biochem. 1979 Jun;57(6):879–888. doi: 10.1139/o79-108. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Byng G. S., Whitaker R. J., Gherna R. L., Jensen R. A. Variable enzymological patterning in tyrosine biosynthesis as a means of determining natural relatedness among the Pseudomonadaceae. J Bacteriol. 1980 Oct;144(1):247–257. doi: 10.1128/jb.144.1.247-257.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byng G. S., Whitaker R. J., Shapiro C. L., Jensen R. A. The aromatic amino acid pathway branches at L-arogenate in Euglena gracilis. Mol Cell Biol. 1981 May;1(5):426–438. doi: 10.1128/mcb.1.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. N., Stanier R. Y., Le Bras G. Regulation of the biosynthesis of amino acids of the aspartate family in Coliform bacteria and Pseudomonads. J Bacteriol. 1969 Sep;99(3):791–801. doi: 10.1128/jb.99.3.791-801.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- Fazel A. M., Bowen J. R., Jensen R. A. Arogenate (pretyrosine) is an obligatory intermediate of L-tyrosine biosynthesis: confirmation in a microbial mutant. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1270–1273. doi: 10.1073/pnas.77.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- GUROFF G., ITO T. PHENYLALANINE HYDROXYLATION BY PSEUDOMONAS SPECIES (ATCC 11299A). J Biol Chem. 1965 Mar;240:1174–1184. [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. C., Jensen R. A. Enzymological basis for growth inhibition by L-phenylalanine in the cyanobacterium Synechocystis sp. 29108. J Bacteriol. 1980 Dec;144(3):1034–1042. doi: 10.1128/jb.144.3.1034-1042.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. C., Jensen R. A. Regulatory isozymes of 3-deoxy-D-arabinoheptulosonate 7-phosphate synthase in the cyanobacterium Anabaena sp. strain ATCC 29151. J Bacteriol. 1981 Oct;148(1):361–364. doi: 10.1128/jb.148.1.361-364.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzclaw W. D., Chapman L. F., Gowans C. S. Some regulatory properties of phospho-2-keto-3-deoxyheptonate aldolase from the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1972 May 12;268(2):562–572. doi: 10.1016/0005-2744(72)90353-1. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Jensen R. A. Growth inhibition by L-phenylalanine in Agmenellum quadruplicatum. A clue to some amino acid interrelationships. Arch Mikrobiol. 1973 Jun 6;91(3):221–233. doi: 10.1007/BF00408909. [DOI] [PubMed] [Google Scholar]
- Jensen R. A. Enzyme recruitment in evolution of new function. Annu Rev Microbiol. 1976;30:409–425. doi: 10.1146/annurev.mi.30.100176.002205. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nester E. W. Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. I. Purification and properties of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. J Biol Chem. 1966 Jul 25;241(14):3365–3372. [PubMed] [Google Scholar]
- Jensen R. A., Stenmark S. L. Comparative allostery of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthetase as a molecular basis for classification. J Bacteriol. 1970 Mar;101(3):763–769. doi: 10.1128/jb.101.3.763-769.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Zamir L., Saint Pierre M., Patel N., Pierson D. L. Isolation and preparation of pretyrosine, accumulated as a dead-end metabolite by Neurospora crassa. J Bacteriol. 1977 Dec;132(3):896–903. doi: 10.1128/jb.132.3.896-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Pierson D. L., Jensen R. A. Dual enzymatic routes to L-tyrosine and L-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J Biol Chem. 1977 Aug 25;252(16):5839–5846. [PubMed] [Google Scholar]
- Raven P. H. A multiple origin for plastids and mitochondria. Science. 1970 Aug 14;169(3946):641–646. doi: 10.1126/science.169.3946.641. [DOI] [PubMed] [Google Scholar]
- Rubin J. L., Jensen R. A. Enzymology of l-Tyrosine Biosynthesis in Mung Bean (Vigna radiata [L.] Wilczek). Plant Physiol. 1979 Nov;64(5):727–734. doi: 10.1104/pp.64.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark S. L., Pierson D. L., Jensen R. A., Glover G. I. Blue-green bacteria synthesise L-tyrosine by the pretyrosine pathway. Nature. 1974 Feb 1;247(5439):290–292. doi: 10.1038/247290a0. [DOI] [PubMed] [Google Scholar]
- WONG P. W., O'FLYNN M. E., INOUYE T. MICROMETHODS FOR MEASURING PHENYLALANINE AND TYROSINE IN SERUM. Clin Chem. 1964 Dec;10:1098–1104. [PubMed] [Google Scholar]
- Whitaker R. J., Byng G. S., Gherna R. L., Jensen R. A. Comparative allostery of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase as an indicator of taxonomic relatedness in pseudomonad genera. J Bacteriol. 1981 Feb;145(2):752–759. doi: 10.1128/jb.145.2.752-759.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


