Abstract
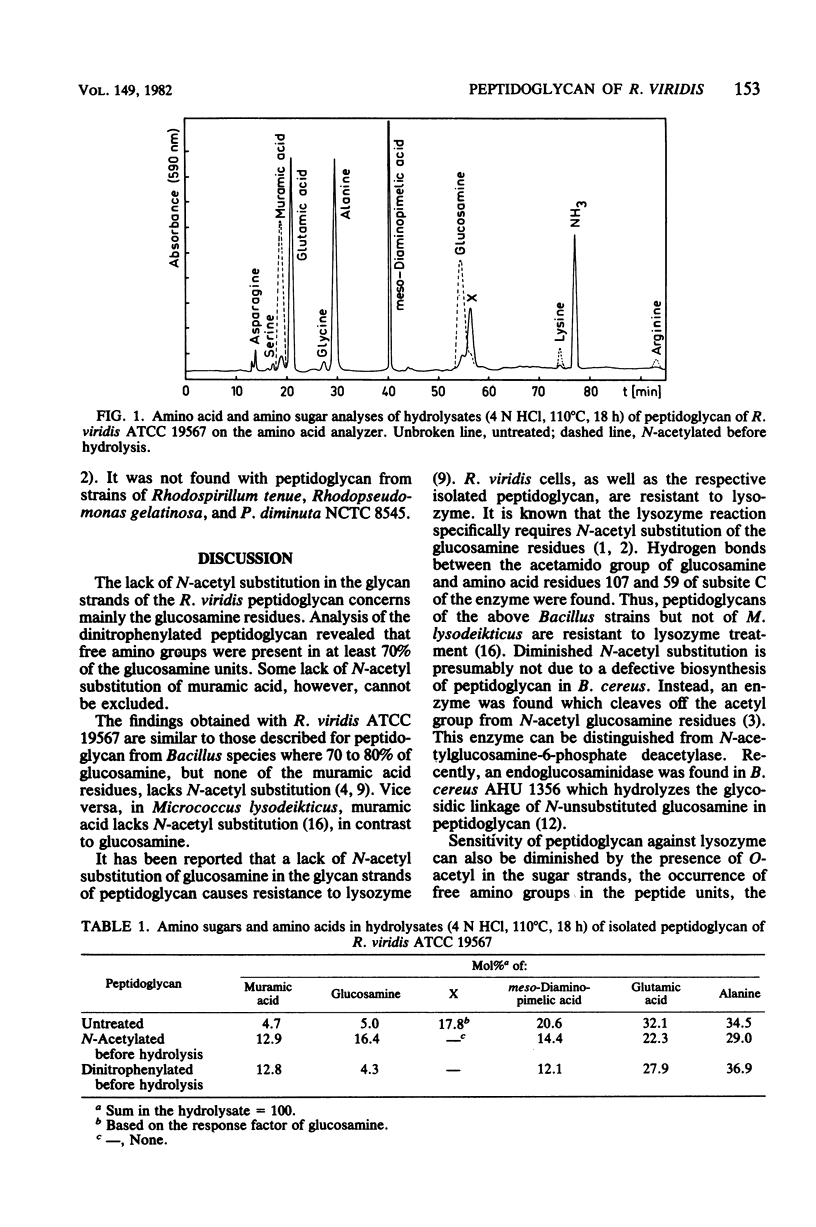
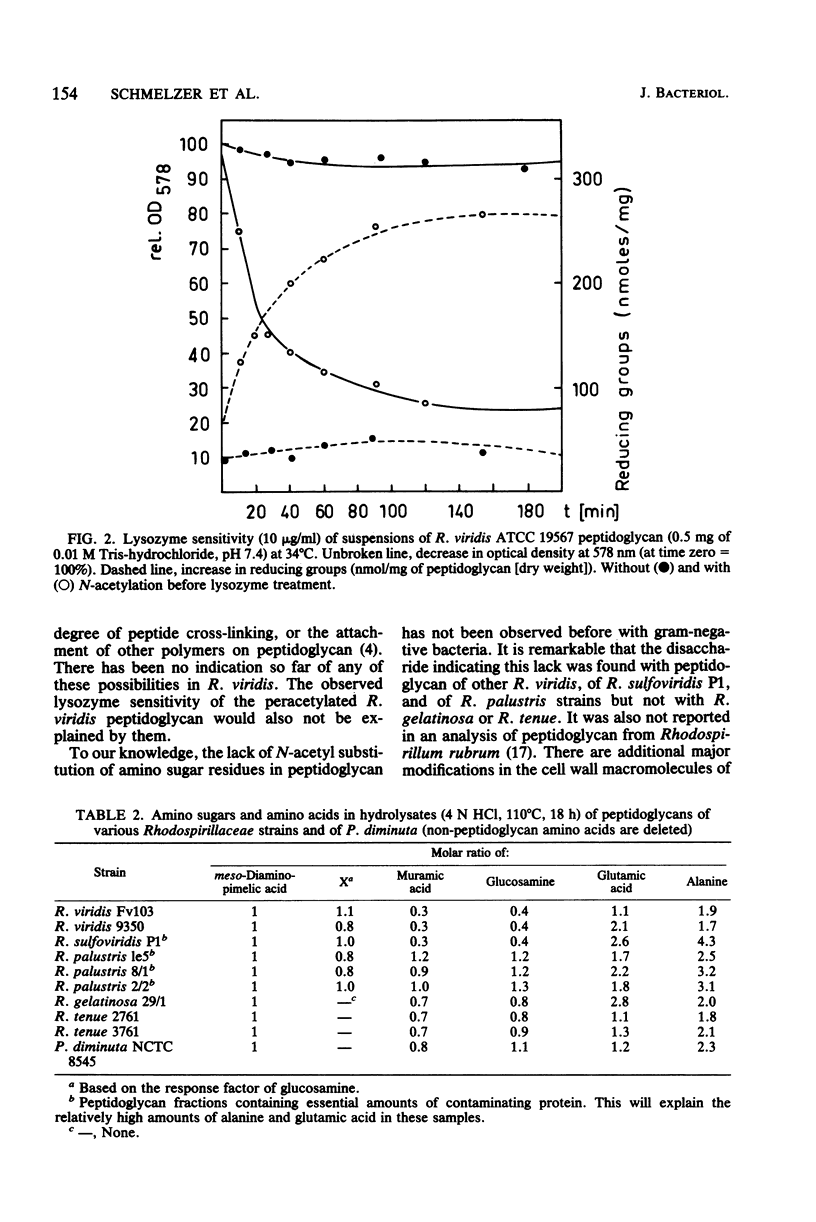
A lack of at least 70% of N-acetyl substitution of glucosamine in the glycan strands of the peptidoglycan from the gram-negative bacterium Rhodopseudomonas viridis is reported. A disaccharide, very likely GlcN beta(1 leads to 4) Mur, was observed in hydrolysates of the isolated peptidoglycan. The disaccharide was not observed when peptidoglycan was N-acetylated before hydrolysis. The peptidoglycan of R. viridis was resistant to lysozyme but became sensitive after N-acetylation with acetic anhydride. The disaccharide was found with peptidoglycan from all R. viridis strains investigated, as well as with R. sulfoviridis P1 and R. palustris strains, but not with peptidoglycan from R. gelatinosa, Rhodospirillum tenue, and Pseudomonas diminuta NCTC 8545.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Araki Y., Ito E. Effect of N-acyl substitution at glucosamine residues on lysozyme-catalyzed hydrolysis of cell-wall peptidoglycan and its oligosaccharides. Eur J Biochem. 1980 Jun;107(2):547–553. doi: 10.1111/j.1432-1033.1980.tb06062.x. [DOI] [PubMed] [Google Scholar]
- Amano K., Hayashi H., Araki Y., Ito E. The action of lysozyme on peptidoglycan with N-unsubstituted glucosamine residues. Isolation of glycan fragments and their susceptibility to lysozyme. Eur J Biochem. 1977 Jun 1;76(1):299–307. doi: 10.1111/j.1432-1033.1977.tb11596.x. [DOI] [PubMed] [Google Scholar]
- Araki Y., Fukuoka S., Oba S., Ito E. Enzymatic deacetylation of N-acetylglucosamine residues in peptidoglycan from Bacillus cereus cell walls. Biochem Biophys Res Commun. 1971 Nov 5;45(3):751–758. doi: 10.1016/0006-291x(71)90481-5. [DOI] [PubMed] [Google Scholar]
- Araki Y., Nakatani T., Nakayama K., Ito E. Occurrence of N-nonsubstituted glucosamine residues in peptidoglycan of lysozyme-resistant cell walls from Bacillus cereus. J Biol Chem. 1972 Oct 10;247(19):6312–6322. [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Fromme I., Beilharz H. Gas chromatographic assay of total and O-acetyl groups in bacterial lipopolysaccharides. Anal Biochem. 1978 Feb;84(2):347–353. doi: 10.1016/0003-2697(78)90051-9. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Araki Y., Ito E. Occurrence of glucosamine residues with free amino groups in cell wall peptidoglycan from bacilli as a factor responsible for resistance to lysozyme. J Bacteriol. 1973 Feb;113(2):592–598. doi: 10.1128/jb.113.2.592-598.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagishi S., Araki Y., Ito E. A novel glycosidase, an endo-glucosaminidase active on the cell wall peptidoglycan with N-unsubstituted glucosamine residues. FEBS Lett. 1979 Jan 1;97(1):20–22. doi: 10.1016/0014-5793(79)80042-3. [DOI] [PubMed] [Google Scholar]
- Keppen O. I., Gorlenko V. M. Kharakteristika novogo vida pochkuiushchikhsia purpurnykh bakterii, soderzhashchikh bakteriokhlorofill b. Mikrobiologiia. 1975 Mar-Apr;44(2):258–264. [PubMed] [Google Scholar]
- MAYER H., RAPIN A. M., KALCKAR H. M. THE SELECTIVITY OF BIOSYNTHESIS OF GLUCOSYL COMPOUNDS AS ILLUSTRATED BY AN E. COLI MUTANT DEFECTIVE IN UDPG SYNTHETASE. Proc Natl Acad Sci U S A. 1965 Feb;53:459–466. doi: 10.1073/pnas.53.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. P., Fleck J., Mock M., Ghuysen J. M. The wall peptidoglycans of Neisseria perflava, Moraxella glucidolytica, Pseudomonas alcaligenes and Proteus vulgaris strain P18. Eur J Biochem. 1973 Oct 5;38(2):301–306. doi: 10.1111/j.1432-1033.1973.tb03062.x. [DOI] [PubMed] [Google Scholar]
- Newton J. W. Linkages in the walls of Rhodospirillum rubrum and its bacilliform mutants. Biochim Biophys Acta. 1968 Oct 15;165(3):534–537. doi: 10.1016/0304-4165(68)90234-1. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Pfennig N. Phototrophic green and purple bacteria: a comparative, systematic survey. Annu Rev Microbiol. 1977;31:275–290. doi: 10.1146/annurev.mi.31.100177.001423. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Drews G., Roppel J., Mayer H., Fromme I. The lipopolysaccharides (O-antigens) of Rhodopseudomonas viridis. Arch Microbiol. 1974;101(3):233–245. doi: 10.1007/BF00455941. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G., Taylor D. P. Occurrence of 2,3-diamino-2,3-dideoxy-d-glucose in lipid A from lipopolysaccharide of pseudomonas diminuta. J Gen Microbiol. 1978 Dec;109(2):367–370. doi: 10.1099/00221287-109-2-367. [DOI] [PubMed] [Google Scholar]


