Abstract
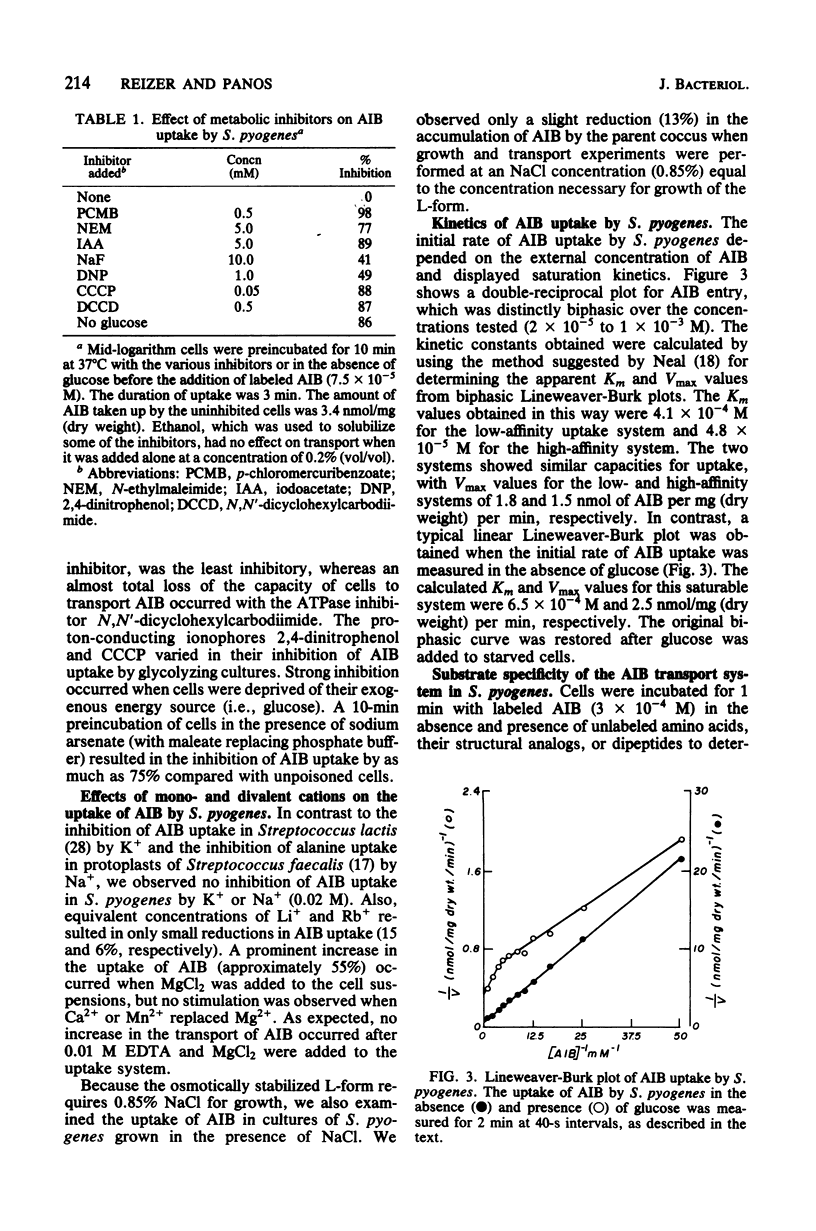
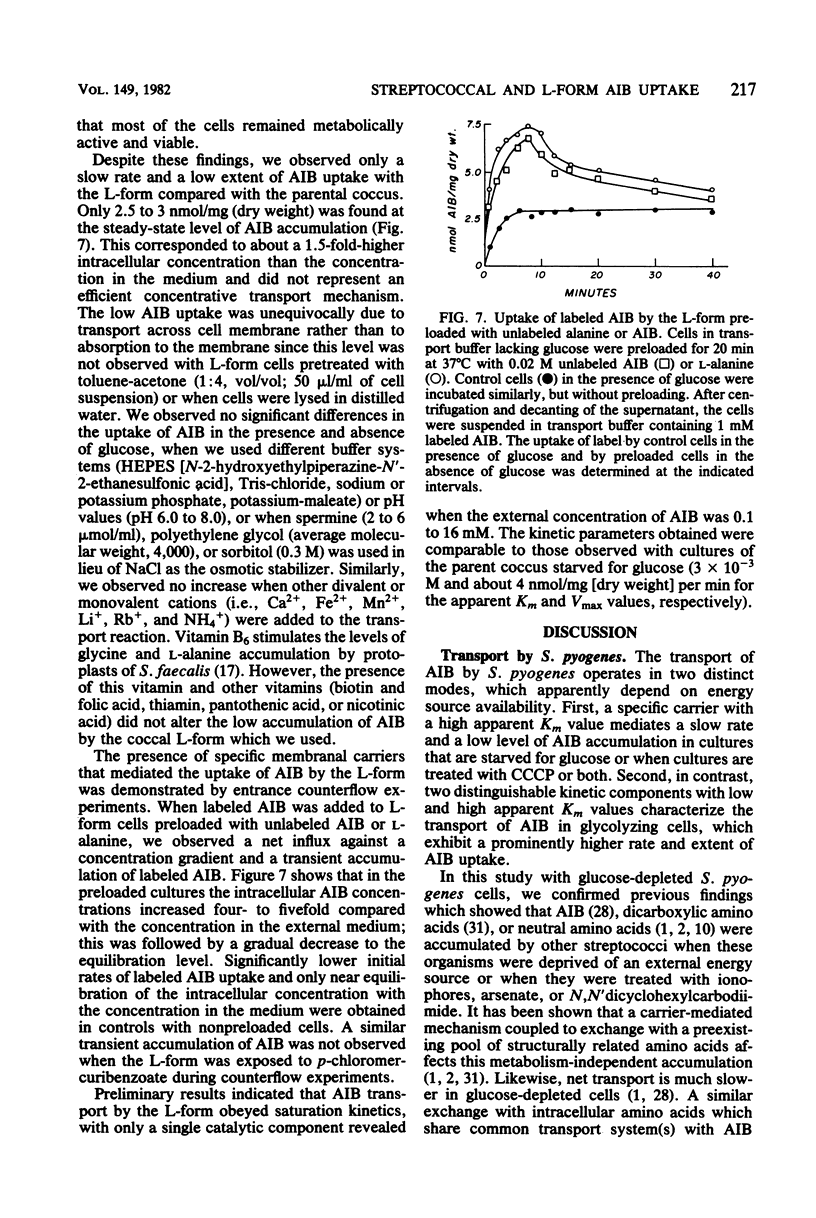
We studied the uptake of α-aminoisobutyric acid (AIB) in Streptococcus pyogenes and its physiologically isotonic L-form. S. pyogenes cells starved for glucose or treated with carbonyl cyanide-m-chlorophenyl hydrazone accumulated limited amounts of AIB. A high apparent Km value characterized the glucose-independent transport of AIB. The rate and extent of AIB accumulation significantly increased in the presence of glucose. Two saturable transport components with distinct apparent Km values characterized glycolysis-coupled transport of AIB. A biphasic Lineweaver-Burk plot was also obtained for l-alanine transport by glycolyzing S. pyogenes cells. AIB seems to share a common transport system(s) with glycine, l- and d-alanine, l-serine, and l-valine. This was shown by the competitive inhibition of AIB uptake by these compounds and their ability to induce competitive exchange efflux of accumulated AIB. About 30% of the AIB uptake was not inhibited by a saturating amount of l-valine, indicating the existence of more than one system for AIB transport. p-Chloromercuribenzoate markedly inhibited the accumulation of AIB by both glycolyzing and glucose-starved cells. In contrast, carbonyl cyanide-m-chlorophenyl hydrazone affected only metabolism-dependent uptake of AIB, which was also sensitive to dinitrophenol, N-ethylmaleimide, iodoacetate, fluoride (NaF), arsenate, and N,N′-dicyclohexylcarbodiimide. These results are interpreted according to the chemiosmotic theory of Mitchell, whereby a proton motive force constitutes the driving force for AIB accumulation. AIB was not accumulated by the L-form. However, a temporary accumulation of AIB by a counterflow mechanism and a saturable system with a low apparent affinity were demonstrated for AIB transport by this organism. We suggest that a deficiency in the coupling of energy to AIB transport is responsible for the apparent lack of active AIB accumulation by the L-form.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- BROCK T. D., MOO-PENN G. An amino acid transport system in Streptococcus faecium. Arch Biochem Biophys. 1962 Aug;98:183–190. doi: 10.1016/0003-9861(62)90171-6. [DOI] [PubMed] [Google Scholar]
- Chevion M., Panos C., Linzer R., Neuhaus F. C. Incorporation of D-alanine into the membrane of Streptococcus pyogenes and its stabilized L-form. J Bacteriol. 1974 Dec;120(3):1026–1032. doi: 10.1128/jb.120.3.1026-1032.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevion M., Panos C., Paxton J. Membrane studies of Streptococcus pyogenes and its L-form growing in hypertonic and physiologically isotonic media. An electron spin resonance spectroscopy approach. Biochim Biophys Acta. 1976 Mar 5;426(2):288–301. doi: 10.1016/0005-2736(76)90338-2. [DOI] [PubMed] [Google Scholar]
- Cohen M., Panos C. Membrane lipid composition of Streptococcus pyogenes and derived L form. Biochemistry. 1966 Jul;5(7):2385–2392. doi: 10.1021/bi00871a031. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Even-Shoshan A. Properties of the glutamate transport system in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1009–1016. doi: 10.1128/jb.93.3.1009-1016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Effects of nigericin and monactin on cation permeability of Streptococcus faecalis and metabolic capacities of potassium-depleted cells. J Bacteriol. 1968 Mar;95(3):816–823. doi: 10.1128/jb.95.3.816-823.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Van Brunt J. Circulation of H+ and K+ across the plasma membrane is not obligatory for bacterial growth. Science. 1977 Jul 22;197(4301):372–373. doi: 10.1126/science.69317. [DOI] [PubMed] [Google Scholar]
- Lenaz G. The role of lipids in the structure and function of membranes. Subcell Biochem. 1979;6:233–343. doi: 10.1007/978-1-4615-7945-8_5. [DOI] [PubMed] [Google Scholar]
- Leon O., Panos C. Adaptation of an osmotically fragile L-form of Streptococcus pyogenes to physiological osmotic conditions and its ability to destroy human heart cells in tissue culture. Infect Immun. 1976 Jan;13(1):252–262. doi: 10.1128/iai.13.1.252-262.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno J. A., Schachter D. Energy-coupled influx of thiomethylgalactoside into Escherichia coli. J Biol Chem. 1970 Mar 10;245(5):1217–1223. [PubMed] [Google Scholar]
- Mason P. W., Carbone D. P., Cushman R. A., Waggoner A. S. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J Biol Chem. 1981 Feb 25;256(4):1861–1866. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in energy transduction: a logical development of biochemical knowledge. J Bioenerg. 1972 May;3(1):5–24. doi: 10.1007/BF01515993. [DOI] [PubMed] [Google Scholar]
- Neal J. L. Analysis of Michaelis kinetics for two independent, saturable membrane transport functions. J Theor Biol. 1972 Apr;35(1):113–118. doi: 10.1016/0022-5193(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Panos C., Cohen M., Fagan G. Lipid alterations after cell wall inhibition. Fatty acid content of Streptococcus pyogenes and derived L-form. Biochemistry. 1966 May;5(5):1461–1468. doi: 10.1021/bi00869a003. [DOI] [PubMed] [Google Scholar]
- Panos C., Fagan G., Zarkadas C. G. Comparative electrophoretic and amino acid analyses of isolated membranes from Streptococcus pyogenes and stabilized L-form. J Bacteriol. 1972 Oct;112(1):285–290. doi: 10.1128/jb.112.1.285-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panos C. STREPTOCOCCAL L-FORMS IV. : Comparison of the Metabolic Rates of a Streptococcus and Derived L-Form. J Bacteriol. 1962 Nov;84(5):921–928. doi: 10.1128/jb.84.5.921-928.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. G., Utech N. M., Holden J. T. Multiple transport components for dicarboxylic amino acids in Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5261–5272. [PubMed] [Google Scholar]
- Reizer J., Panos C. Regulation of beta-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5497–5501. doi: 10.1073/pnas.77.9.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch V. M., Panos C. Defective synthesis of lipid intermediates for peptidoglycan formation in a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1976 Apr;126(1):300–311. doi: 10.1128/jb.126.1.300-311.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Turner K. W., Thomas T. D. Catabolite inhibition and sequential metabolism of sugars by Streptococcus lactis. J Bacteriol. 1978 Mar;133(3):1163–1174. doi: 10.1128/jb.133.3.1163-1174.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toci R., Belaich A., Belaich J. P. Influence of "energization" on the binding of M protein with p-nitrophenyl alpha-D-galactopyranoside. J Biol Chem. 1980 May 25;255(10):4603–4606. [PubMed] [Google Scholar]
- Utech N. M., Reid K. G., Holden J. T. Properties of a dicarboxylic amino acid transport-deficient mutant of Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5273–5280. [PubMed] [Google Scholar]
- Wong P. T., MacLennan D. H. Restoration by fatty acids of active transport in a lactose transport mutant of Escherichia coli. Can J Biochem. 1973 May;51(5):538–549. doi: 10.1139/o73-067. [DOI] [PubMed] [Google Scholar]


