Abstract
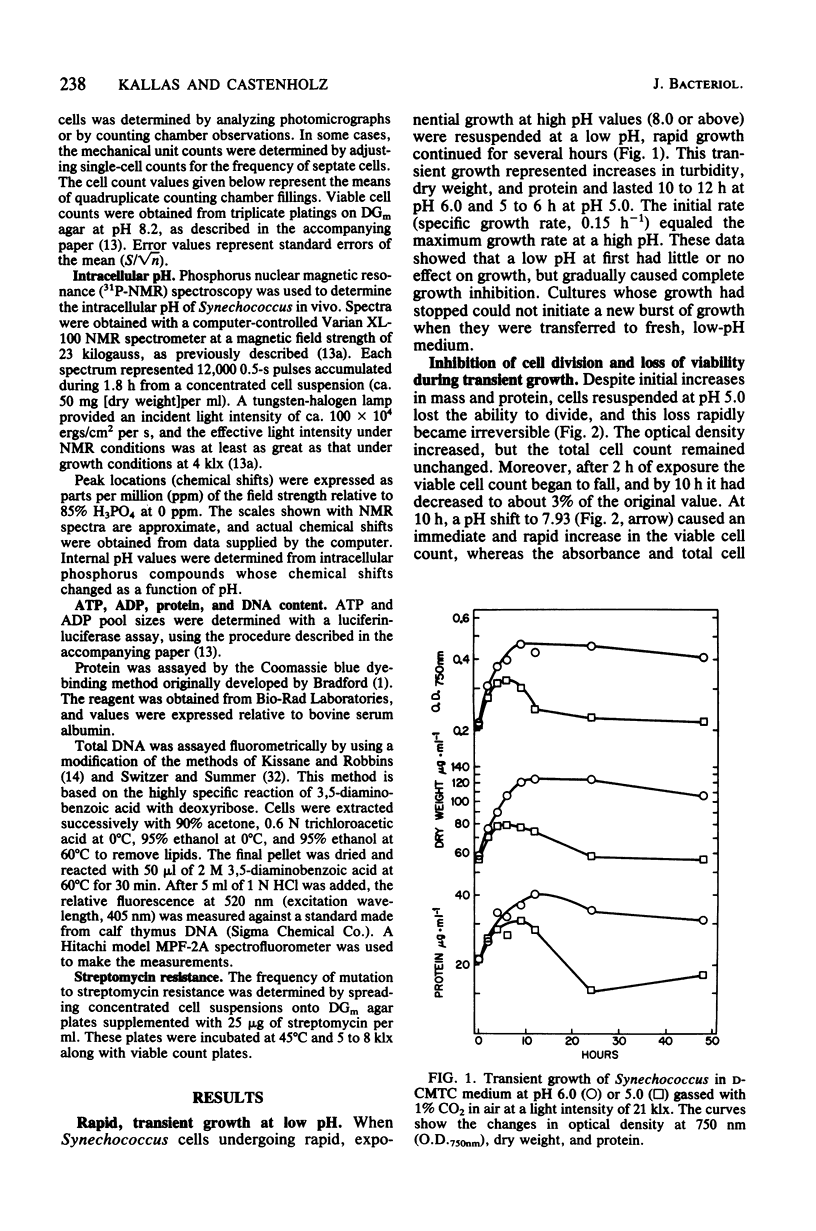
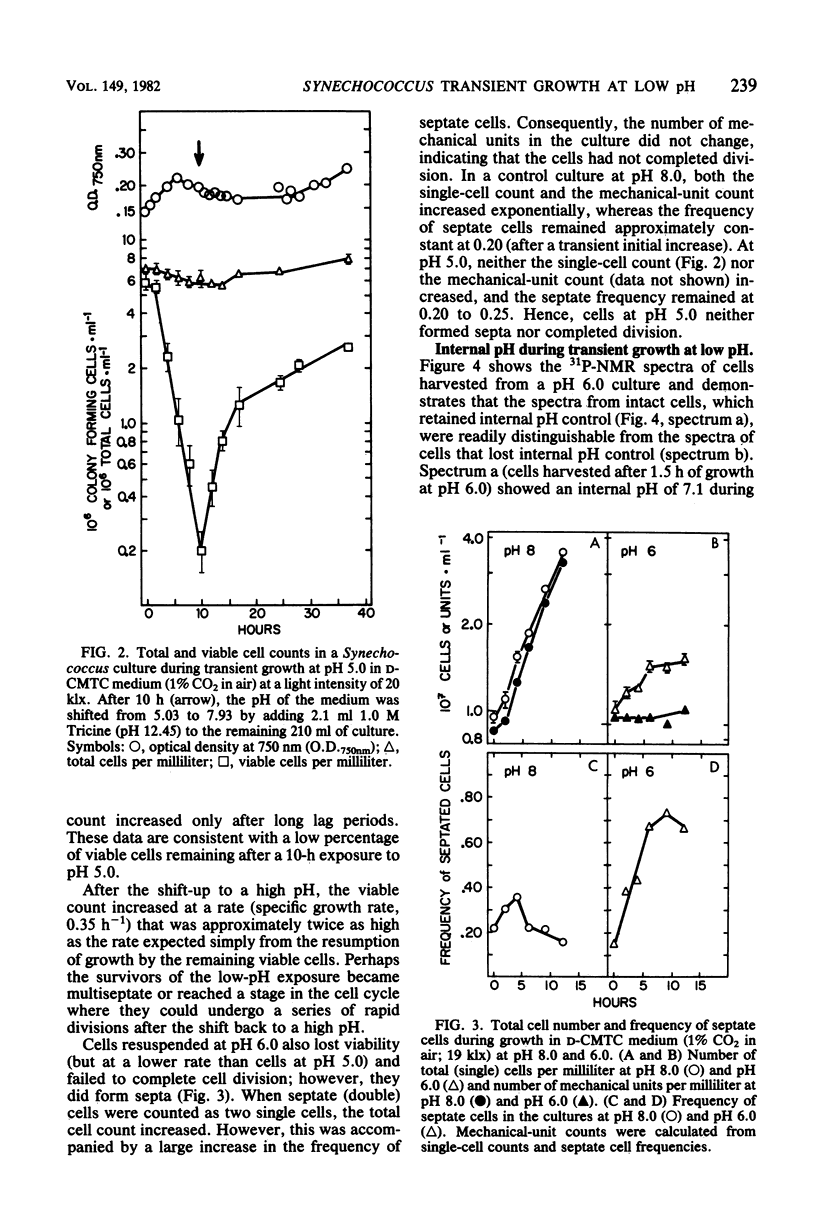
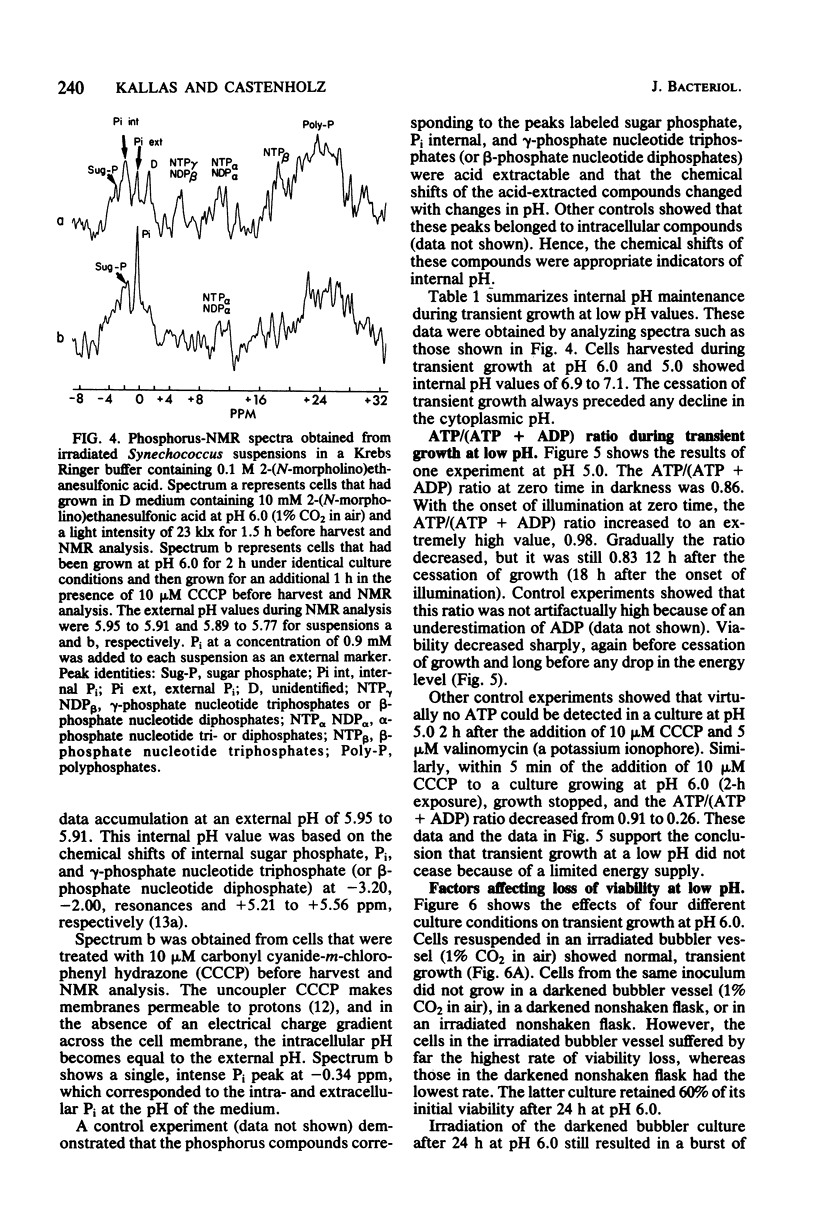
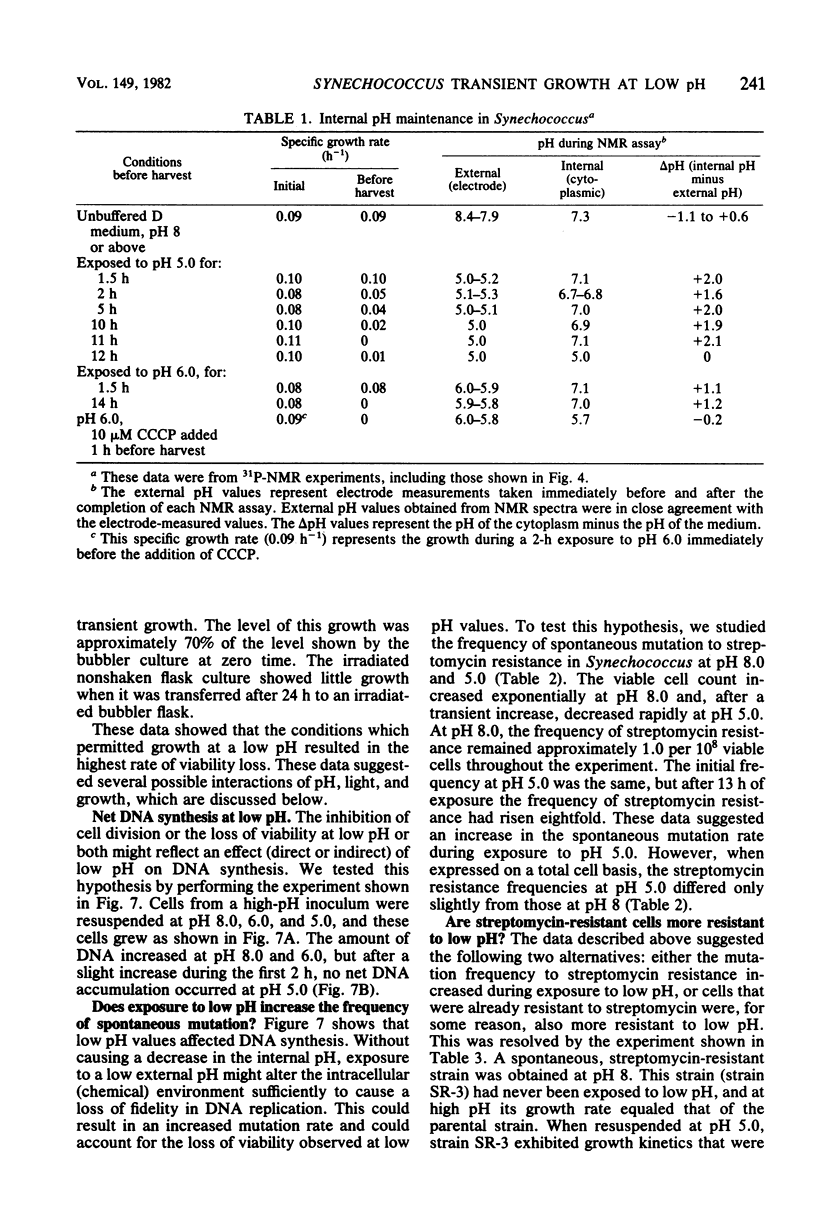
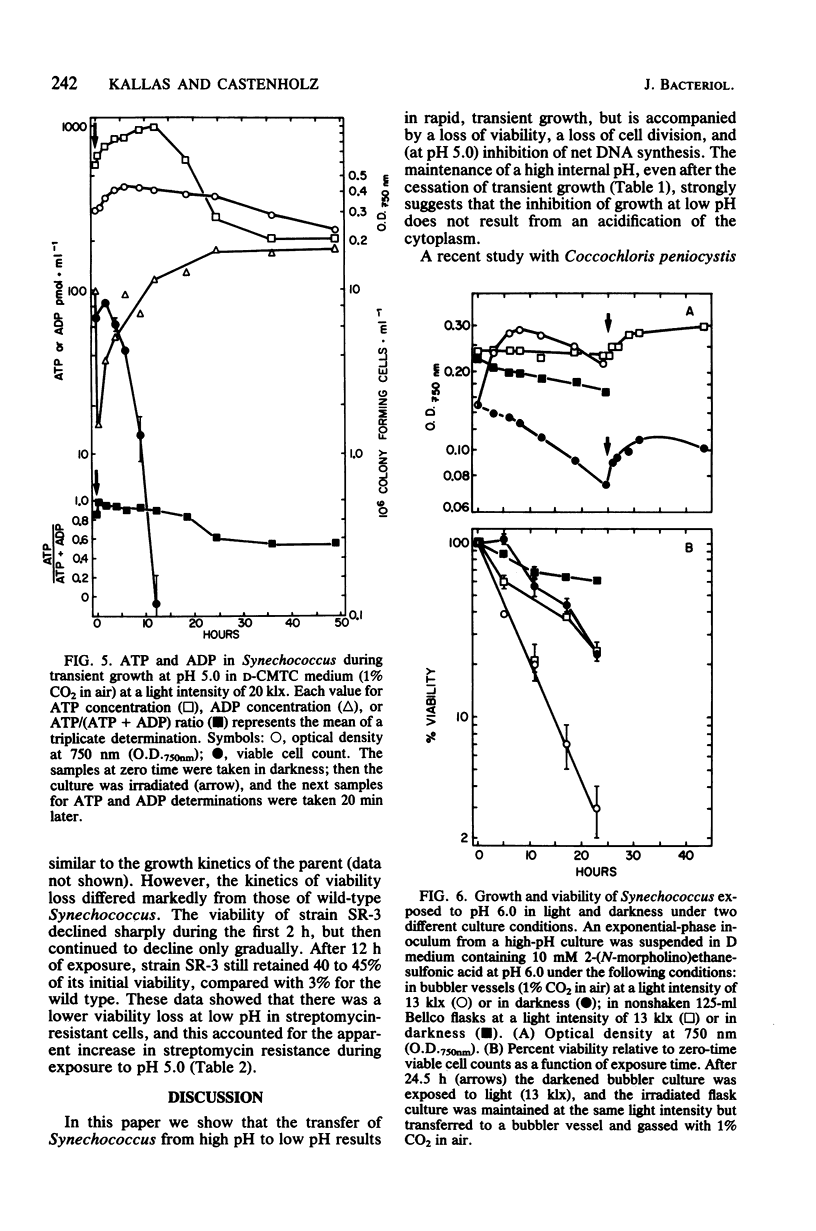
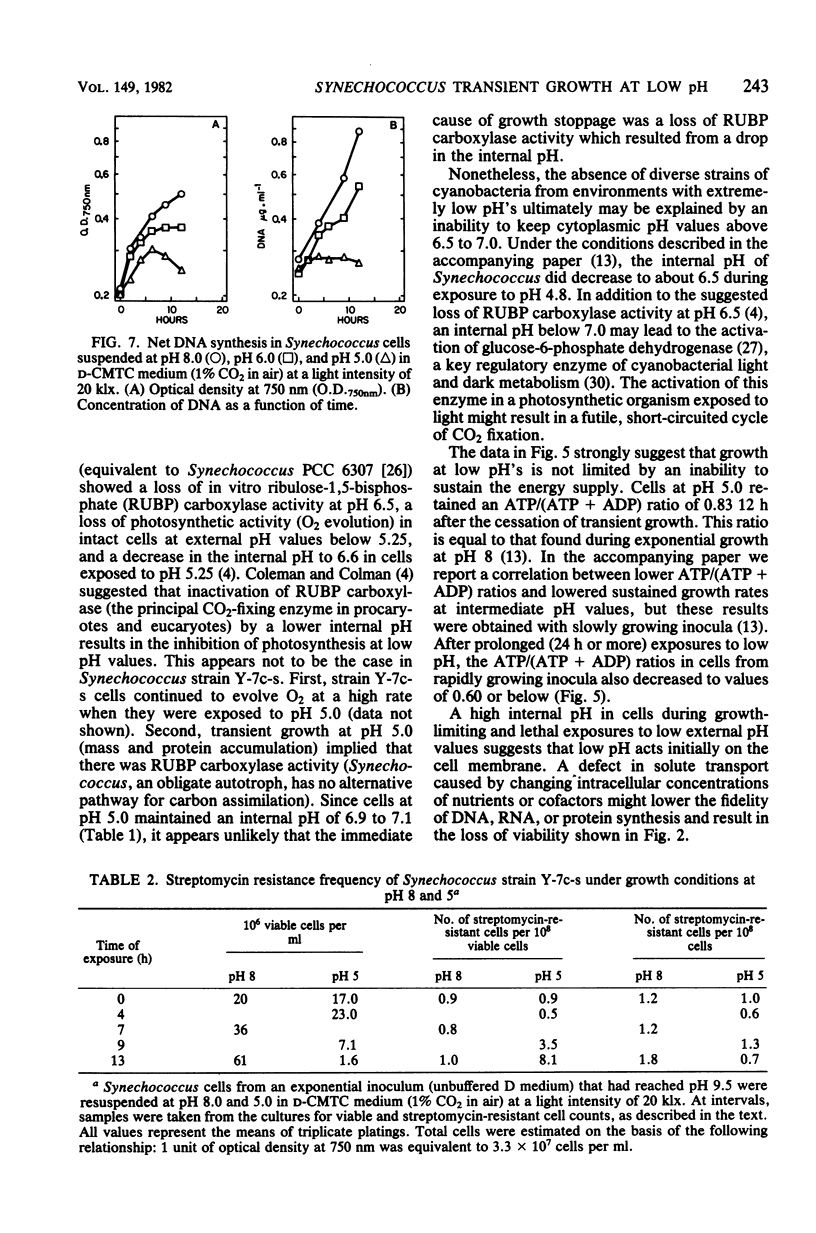
The thermophilic cyanobacterium Synechococcus sp. strain Y-7c-s grows at its maximum rate at a high pH (pH 8 and above) the does not show sustained growth below pH 6.5. However, rapidly growing, exponential-phase cells from high-pH cultures continued to grow rapidly for several hours after transfer to pH 6.0 or 5.0. This transient growth represented increases in mass and protein, but cells failed to complete division. Viability loss commenced well before the cessation of growth, and cells at pH 5.0 showed no net DNA synthesis. When irradiated by visible light, cells at pH 6.0 and 5.0 maintained and internal pH of 6.9 to 7.1 (determined by 31P nuclear magnetic resonance spectroscopy) and an extremely high ATP/(ATP + ADP) ratio even after growth had ceased. Cells exposed to a low pH did not show an increase in the spontaneous mutation rate, as measured by mutation to streptomycin resistance. However, cells already resistant to streptomycin were more resistant to viability loss at a low pH than the parental type. Cultures that could grow transiently at a low pH had higher rates of viability loss than nongrowing cultures in light or darkness. The retention of a high internal pH by cells exposed to a low pH suggested that a low pH acted initially on the cell membrane, possibly on solute transport.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brey R. N., Rosen B. P., Sorensen E. N. Cation/proton antiport systems in Escherichia coli. Properties of the potassium/proton antiporter. J Biol Chem. 1980 Jan 10;255(1):39–44. [PubMed] [Google Scholar]
- Brock T. D. Lower pH limit for the existence of blue-green algae: evolutionary and ecological implications. Science. 1973 Feb 2;179(4072):480–483. doi: 10.1126/science.179.4072.480. [DOI] [PubMed] [Google Scholar]
- Coleman J. R., Colman B. Inorganic Carbon Accumulation and Photosynthesis in a Blue-green Alga as a Function of External pH. Plant Physiol. 1981 May;67(5):917–921. doi: 10.1104/pp.67.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Lutkenhaus J. F., Salmond G. P., Martinez-Salas E., Vincente M. Role of the ftsA gene product in control of Escherichia coli cell division. J Bacteriol. 1979 Nov;140(2):388–394. doi: 10.1128/jb.140.2.388-394.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOGG G. E. The comparative physiology and biochemistry of the blue-green algae. Bacteriol Rev. 1956 Sep;20(3):148–165. doi: 10.1128/br.20.3.148-165.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Susman P., Blanco R., Krulwich T. A. The protonmotive force and alpha-aminoisobutyric acid transport in an obligately alkalophilic bacterium. J Biol Chem. 1978 Feb 10;253(3):708–715. [PubMed] [Google Scholar]
- Harold F. M. Ion currents and physiological functions in microorganisms. Annu Rev Microbiol. 1977;31:181–203. doi: 10.1146/annurev.mi.31.100177.001145. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kallas T., Dahlquist F. W. Phosphorus-31 nuclear magnetic resonance analysis of internal pH during photosynthesis in the cyanobacterium Synechococcus. Biochemistry. 1981 Sep 29;20(20):5900–5907. doi: 10.1021/bi00523a038. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Davidson L. F., Filip S. J., Jr, Zuckerman R. S., Guffanti A. A. The protonmotive force and beta-galactoside transport in Bacillus acidocaldarius. J Biol Chem. 1978 Jul 10;253(13):4599–4603. [PubMed] [Google Scholar]
- Krulwich T. A., Mandel K. G., Bornstein R. F., Guffanti A. A. A non-alkalophilic mutant of Bacillus alcalophilus lacks the Na+/H+ antiporter. Biochem Biophys Res Commun. 1979 Nov 14;91(1):58–62. doi: 10.1016/0006-291x(79)90582-5. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Coupling of aspartate and serine transport to the transmembrane electrochemical gradient for sodium ions in Halobacterium halobium. Translocation stoichiometries and apparent cooperativity. Biochemistry. 1978 Jul 25;17(15):3011–3018. doi: 10.1021/bi00608a012. [DOI] [PubMed] [Google Scholar]
- Mann N., Carr N. G. Coupling between the initiation of DNA replication and cell division in the blue-green alga Anacystis nidulans. Arch Microbiol. 1977 Feb 4;112(1):95–98. doi: 10.1007/BF00446659. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., Komano T., Maruyama Y. Cell-cycle-specific inhibition by chloramphenicol of septum fromation and cell division in synchronized cells of Bacillus subtilis. J Bacteriol. 1980 Feb;141(2):502–507. doi: 10.1128/jb.141.2.502-507.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Rhodopseudomonas acidophila, sp. n., a new species of the budding purple nonsulfur bacteria. J Bacteriol. 1969 Aug;99(2):597–602. doi: 10.1128/jb.99.2.597-602.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack R. H., Jr, Rosen B. P. Cation/proton antiport systems in Escherichia coli. Absence of potassium/proton antiporter activity in a pH-sensitive mutant. J Biol Chem. 1980 May 10;255(9):3824–3825. [PubMed] [Google Scholar]
- Raboy B., Padan E. Active transport of glucose and alpha-methylglucoside in the cyanobacterium Plectonema boryanum. J Biol Chem. 1978 May 10;253(9):3287–3291. [PubMed] [Google Scholar]
- Schaeffer F., Stanier R. Y. Glucose-6-phosphate dehydrogenase of Anabaena sp. Kinetic and molecular properties. Arch Microbiol. 1978 Jan 23;116(1):9–19. doi: 10.1007/BF00408728. [DOI] [PubMed] [Google Scholar]
- Stevens S. E., Porter R. D. Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6052–6056. doi: 10.1073/pnas.77.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer B. R., Summer G. K. A modified fluorometric micromethod for DNA. Clin Chim Acta. 1971 Apr;32(2):203–206. doi: 10.1016/0009-8981(71)90333-0. [DOI] [PubMed] [Google Scholar]


