Abstract
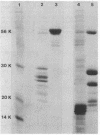
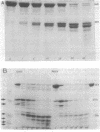
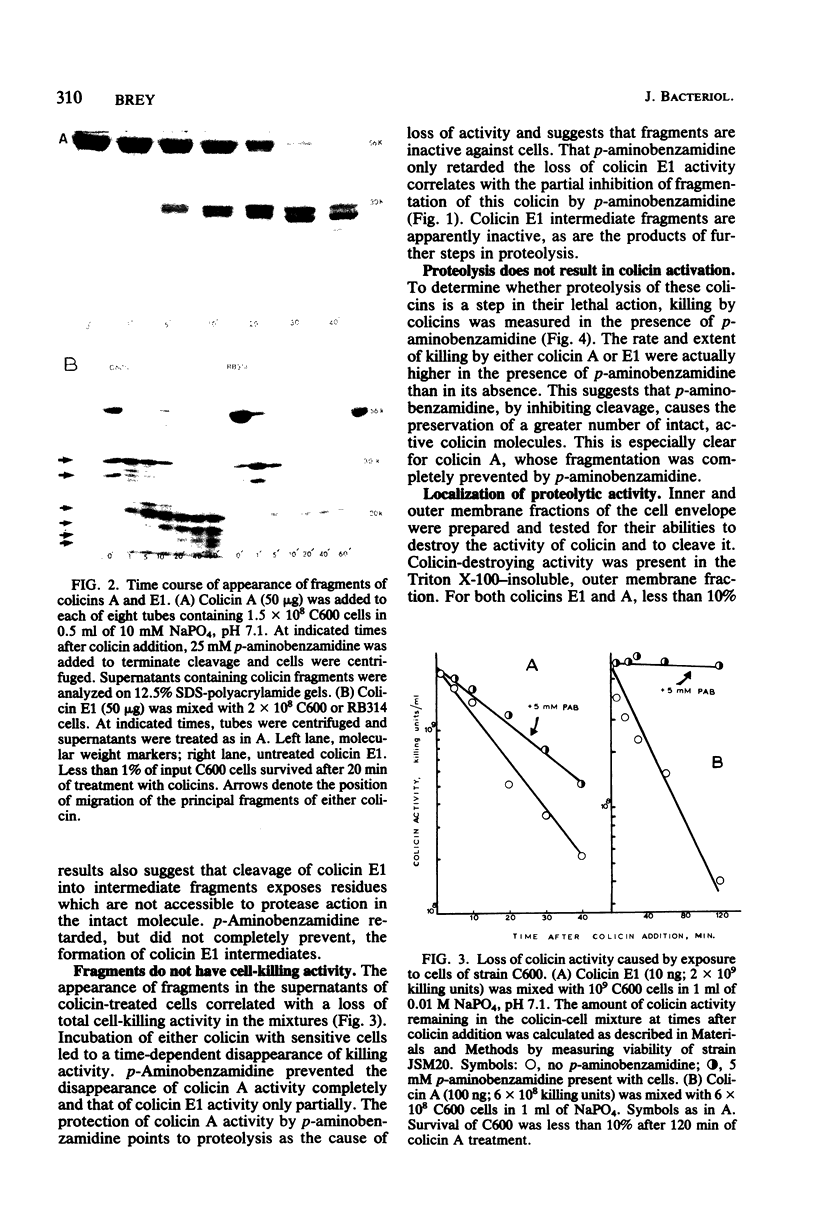
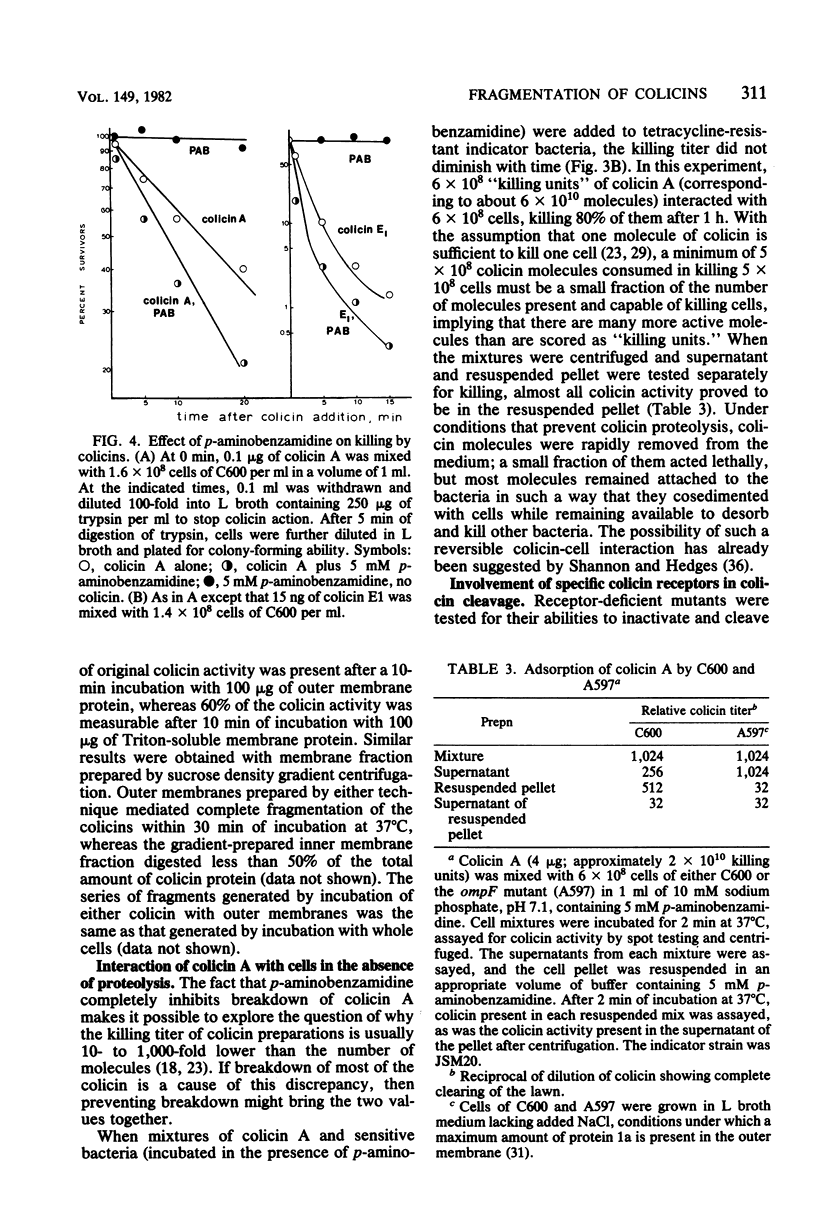
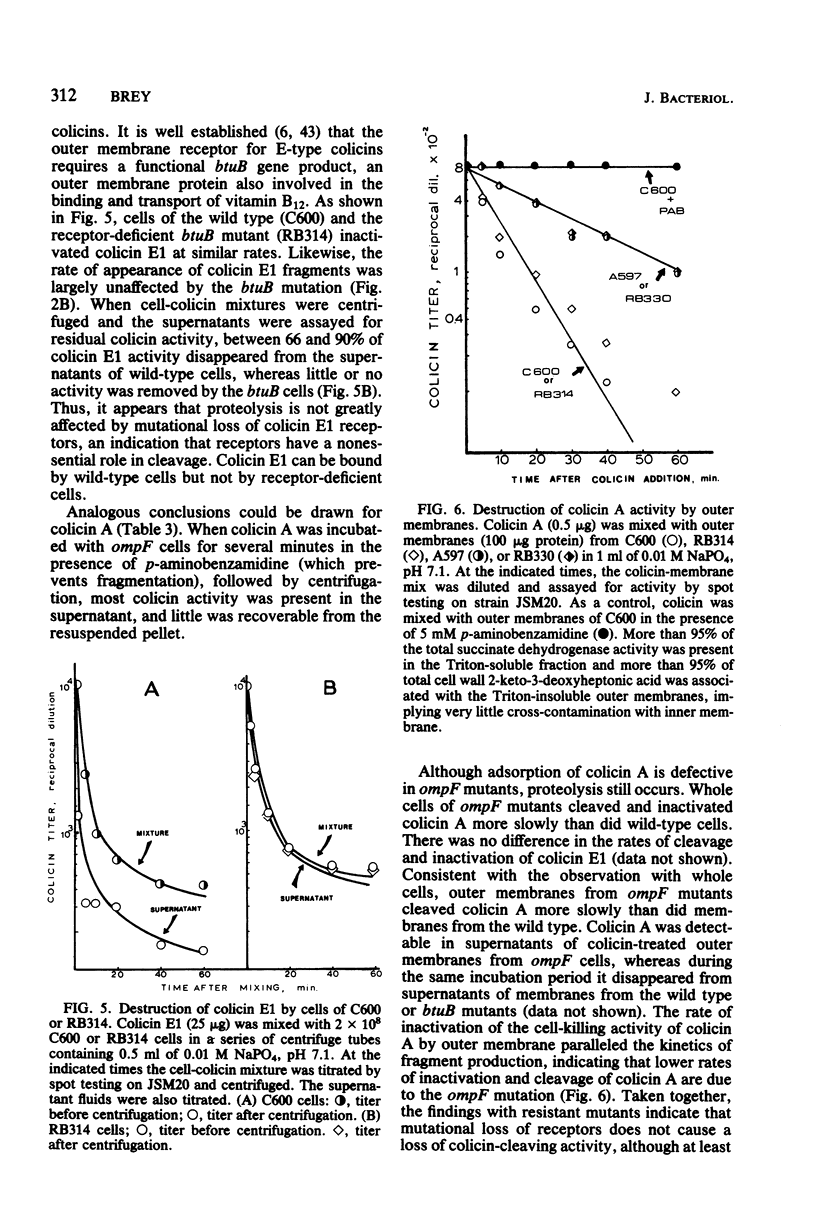
Interaction of either colicin A or E1 with the surface of Escherichia coli cells resulted in extensive cleavage of the colicins into many peptide fragments in the molecular weight range of 10,000 to 30,000 released into the supernatants of colicin-cell mixtures. The protease inhibitor P-aminobenzamidine inhibited the cleavage of colicin A and enhanced colicin killing activity, suggesting that proteolysis is not required for the killing action of colicin. Fragments derived from the supernatants of the mixtures were inactive against sensitive cells. Proteolytic activity against both colicins was localized primarily in the outer membrane fraction of the cell envelope. At least two distinct protease activities appear to be present. Examination of the patterns of cleavage and inactivation of the colicins by a series of resistant mutants indicates that specific colicin receptors play no essential role in colicin proteolysis. In addition, evidence is presented that adsorption of colicin to specific receptors is a reversible process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles L. K., Konisky J. Cleavage of colicin Ia by the Escherichia coli K-12 outer membrane is not mediated by the colicin Ia receptor. J Bacteriol. 1981 Jan;145(1):668–671. doi: 10.1128/jb.145.1.668-671.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich D. L., Summers A. O., Schnaitman C. A. Outer membrane proteins of Escherichia coli. V. Evidence that protein 1 and bacteriophage-directed protein 2 are different polypeptides. J Bacteriol. 1977 Aug;131(2):598–607. doi: 10.1128/jb.131.2.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lau C., Richards F. M. Behavior of colicins E1, E2, and E3 attached to sephadex beads. Biochemistry. 1976 Feb 10;15(3):666–671. doi: 10.1021/bi00648a034. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H., Bishop C. W., Blech J. E. Localization of proteolytic activity in the outer membrane of Escherichia coli. J Bacteriol. 1979 Jan;137(1):574–583. doi: 10.1128/jb.137.1.574-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A., Nomura M. Interaction of colicins with bacterial cells. I. Studies with radioactive colicins. J Bacteriol. 1966 Feb;91(2):685–694. doi: 10.1128/jb.91.2.685-694.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel G., Wickner W. Translational and post-translational cleavage of M13 procoat protein: extracts of both the cytoplasmic and outer membranes of Escherichia coli contain leader peptidase activity. Proc Natl Acad Sci U S A. 1979 Jan;76(1):236–240. doi: 10.1073/pnas.76.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Voellmy R., Goldberg A. L. Protein degradation is stimulated by ATP in extracts of Escherichia coli. J Biol Chem. 1979 Sep 10;254(17):8194–8200. [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Ohno S., Ohno-Iwashita Y., Suzuki K., Imahori K. Purification and characterization of active component and active fragment of colicin E3. J Biochem. 1977 Oct;82(4):1045–1053. doi: 10.1093/oxfordjournals.jbchem.a131775. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Plate C. A. Requirement for membrane potential in active transport of glutamine by Escherichia coli. J Bacteriol. 1979 Jan;137(1):221–225. doi: 10.1128/jb.137.1.221-225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Purification and properties of the colicin E3 receptor of Escherichia coli. J Biol Chem. 1973 Mar 10;248(5):1797–1806. [PubMed] [Google Scholar]
- Sato T., Yura T. Regulatory mutations conferring constitutive synthesis of major outer membrane proteins (OmpC and OmpF) in Escherichia coli. J Bacteriol. 1981 Jan;145(1):88–96. doi: 10.1128/jb.145.1.88-96.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller K., Nomura M. Colicin E2 is DNA endonuclease. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3989–3993. doi: 10.1073/pnas.73.11.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Kagan B. L., Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978 Nov 9;276(5684):159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Shannon R., Hedges A. J. Reversibility of the specific adsorption of colicin E2-P9 to cells of colicin-sensitive strains of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1136–1144. doi: 10.1128/jb.116.3.1136-1144.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. Effect of colicins Ia and E1 on ion permeability of liposomes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6167–6171. doi: 10.1073/pnas.76.12.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. In vitro depolarization of Escherichia coli membrane vesicles by colicin Ia. J Biol Chem. 1978 Nov 10;253(21):7731–7737. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Watson D. H., Sherratt D. J. In vivo proteolytic cleavage of colicins requires specific receptor binding. Nature. 1979 Mar 22;278(5702):362–364. doi: 10.1038/278362a0. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Luria S. E. Reduction of membrane potential, an immediate effect of colicin K. Proc Natl Acad Sci U S A. 1978 May;75(5):2483–2487. doi: 10.1073/pnas.75.5.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. C., DiGirolamo P. M., Fu M. L., Preston Y. A., Bradbeer C. Transport of vitamin B 12 in Escherichia coli. Location and properties of the initial B 12 -binding site. J Biol Chem. 1973 Jun 10;248(11):3978–3986. [PubMed] [Google Scholar]
- de Graaf F. K., Stukart M. J., Boogerd F. C., Metselaar K. Limited proteolysis of cloacin DF13 and characterization of the cleavage products. Biochemistry. 1978 Mar 21;17(6):1137–1142. doi: 10.1021/bi00599a031. [DOI] [PubMed] [Google Scholar]