Abstract
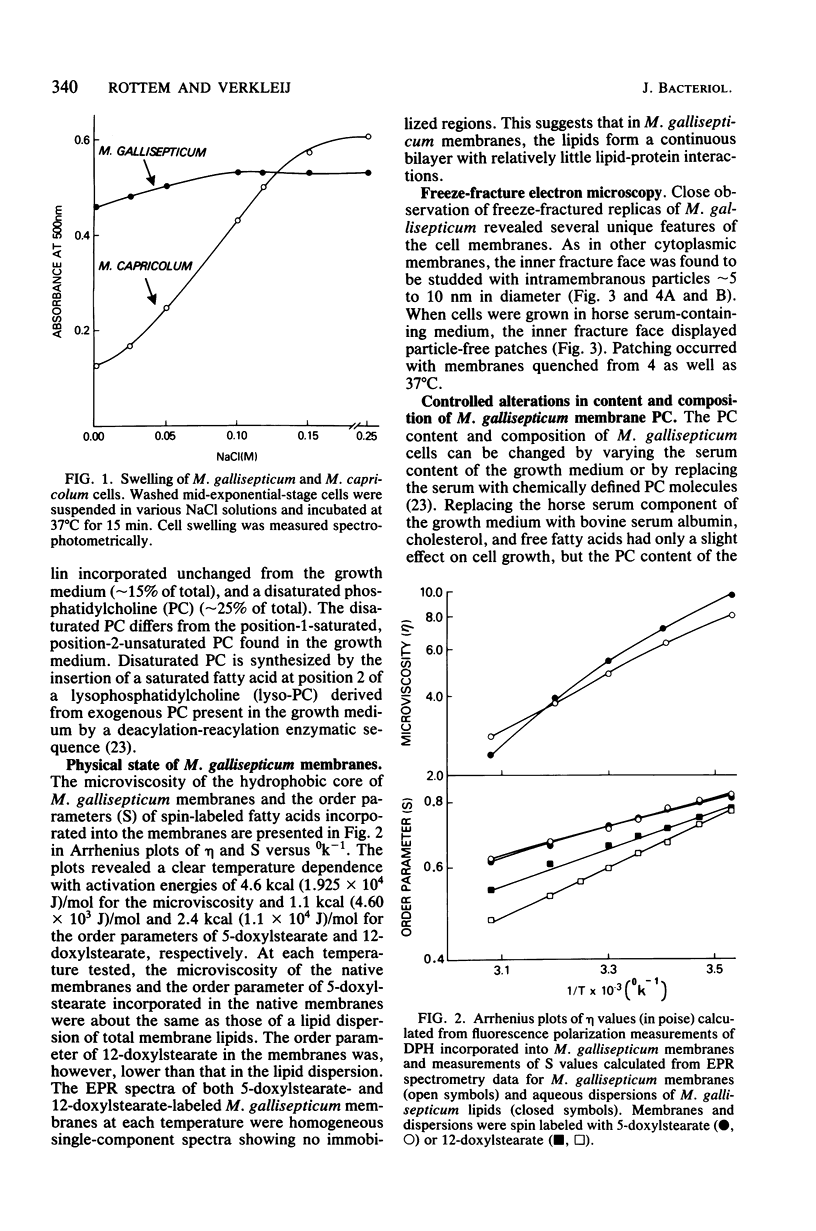
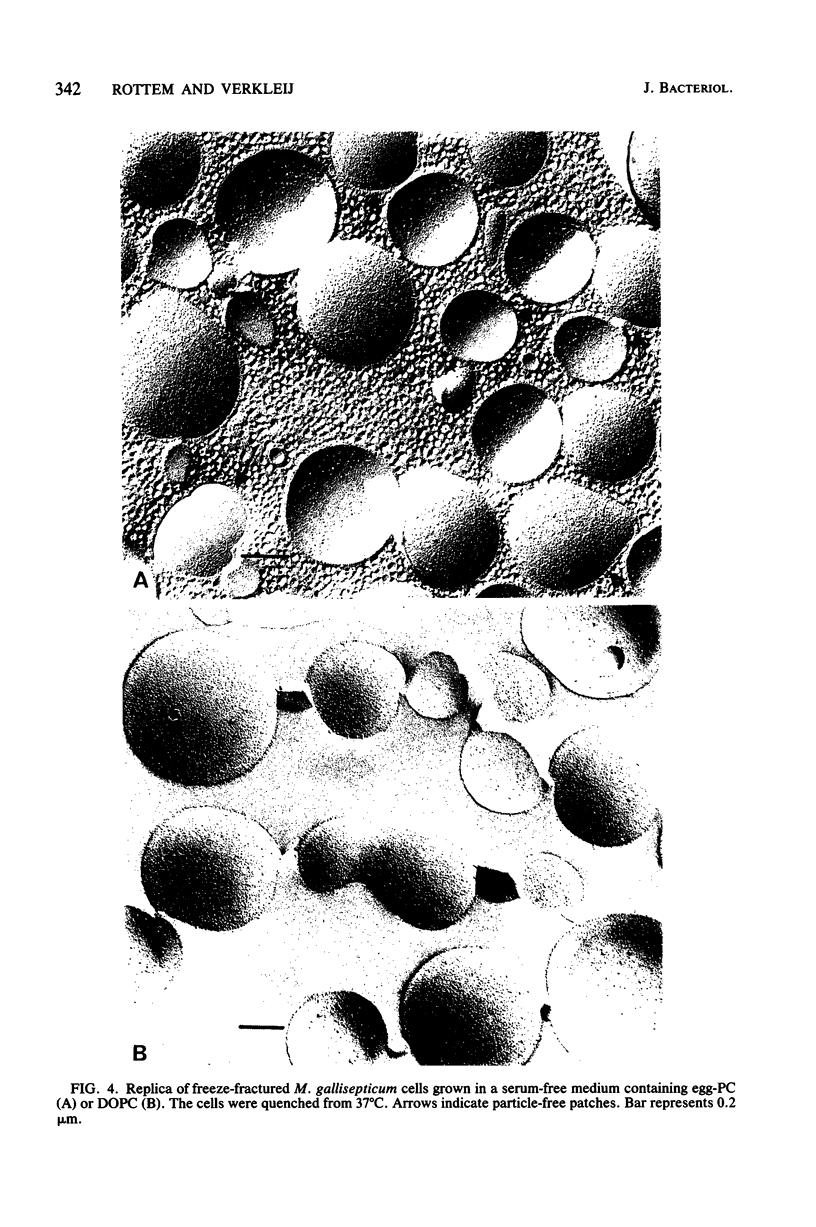
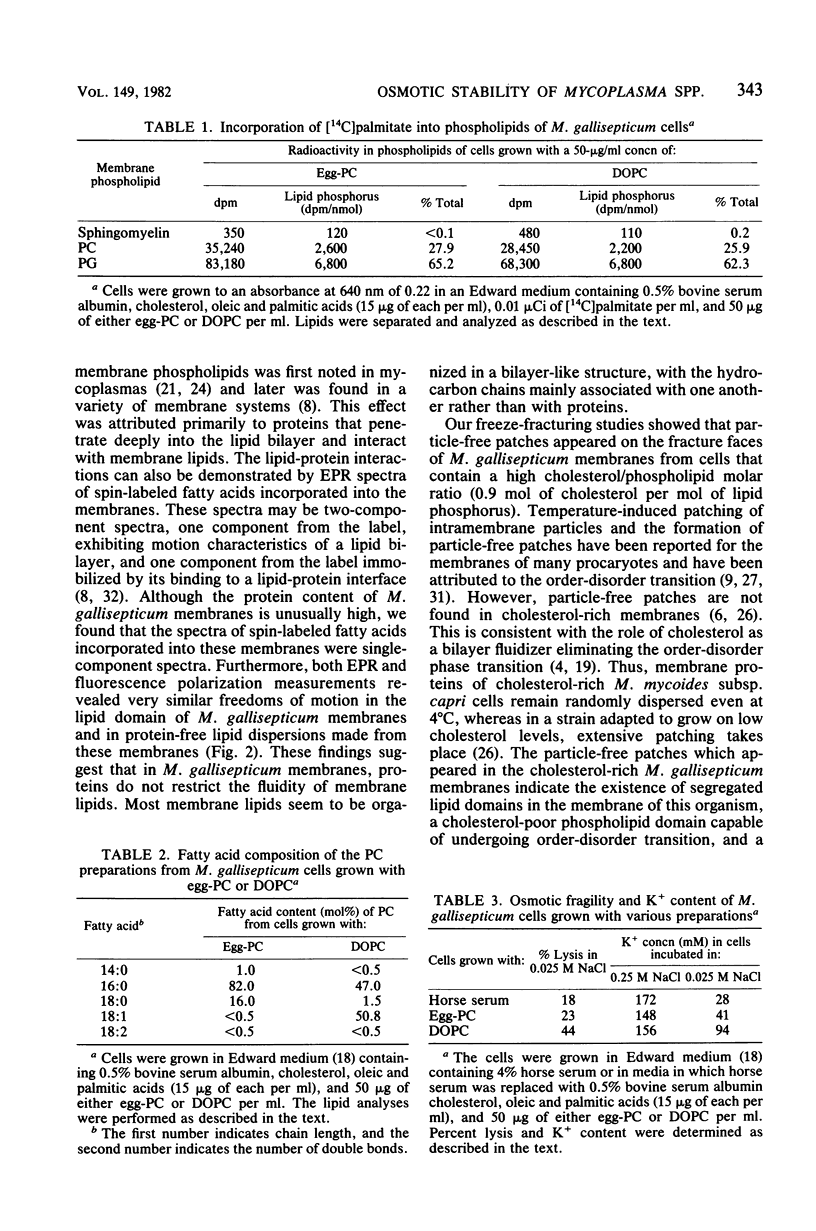
Freeze-fracturing of cholesterol-rich Mycoplasma gallisepticum membranes from cells grown in a medium containing horse serum revealed particle-free patches. The patches appeared in cells quenched from either 4 or 37 degrees C. Particle-free patches also occurred in membranes of cells grown in a serum-free medium supplemented with egg-phosphatidylcholine but not in membranes of cells grown with dioleoylphosphatidylcholine. The appearance of particle-free patches was attributed to the presence of disaturated phosphatidylcholine (PC) molecules in M. gallisepticum membranes, which were synthesized by the insertion of a saturated fatty acid at position 2 of lysophosphatidylcholine derived from exogenous PC present in the growth medium. Consequences of the synthesis of the disaturated PC also included a decrease in osmotic fragility and the ability of the cells to be permeated by K+. Electron paramagnetic resonance and fluorescence polarization measurements revealed that the fluidity of the lipid domain in the protein-rich M. gallisepticum membranes was almost identical to that of an aqueous dispersion of M. gallisepticum membrane lipids. Furthermore, the electron paramagnetic resonance spectra of the membranes were single-component spectra showing no indication of immobilized regions. The possibility that the osmotic resistance of M. gallisepticum cells is associated with the particle-free patches rather than with a restricted membrane fluidity caused by membrane proteins is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blok M. C., van der Neut-Kok E. C., van Deenen L. L., de Gier J. The effect of chain length and lipid phase transitions on the selective permeability properties of liposomes. Biochim Biophys Acta. 1975 Oct 6;406(2):187–196. doi: 10.1016/0005-2736(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Chapman D. Liquid crystals and cell membranes. Ann N Y Acad Sci. 1966 Jul 14;137(2):745–754. doi: 10.1111/j.1749-6632.1966.tb50196.x. [DOI] [PubMed] [Google Scholar]
- Duppel W., Dahl G. Effect of phase transition on the distribution of membrane-associated particles in microsomes. Biochim Biophys Acta. 1976 Mar 19;426(3):408–417. doi: 10.1016/0005-2736(76)90386-2. [DOI] [PubMed] [Google Scholar]
- Gaffney B. J. Fatty acid chain flexibility in the membranes of normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1975 Feb;72(2):664–668. doi: 10.1073/pnas.72.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haest C. W., Verkleij A. J., De Gier J., Scheek R., Ververgaert P. H., Van Deenen L. L. The effect of lipid phase transitions on the architecture of bacterial membranes. Biochim Biophys Acta. 1974 Jul 12;356(1):17–26. doi: 10.1016/0005-2736(74)90290-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marsh D., Watts A., Knowles P. F. Evidence for phase boundary lipid. Permeability of Tempo-choline into dimyristoylphosphatidylcholine vesicles at the phase transition. Biochemistry. 1976 Aug 10;15(16):3570–3578. doi: 10.1021/bi00661a027. [DOI] [PubMed] [Google Scholar]
- Mcelhaney R. N., de Gier J., van der Neut-Kok E. C. The effect of alterations in fatty acid composition and cholesterol content on the nonelectrolyte permeability of Acholeplasma laidlawii B cells and derived liposomes. Biochim Biophys Acta. 1973 Mar 16;298(2):500–512. doi: 10.1016/0005-2736(73)90376-3. [DOI] [PubMed] [Google Scholar]
- Melchior D. L., Steim J. M. Thermotropic transitions in biomembranes. Annu Rev Biophys Bioeng. 1976;5:205–238. doi: 10.1146/annurev.bb.05.060176.001225. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul 6;311(3):330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Rottem S., Hubbell W. L., Hayflick L., McConnell H. M. Motion of fatty acid spin labels in the plasma membrane of mycoplasma. Biochim Biophys Acta. 1970;219(1):104–113. doi: 10.1016/0005-2736(70)90065-9. [DOI] [PubMed] [Google Scholar]
- Rottem S., Linker C., Wilson T. H. Proton motive force across the membrane of Mycoplasma gallisepticum and its possible role in cell volume regulation. J Bacteriol. 1981 Mar;145(3):1299–1304. doi: 10.1128/jb.145.3.1299-1304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Markowitz O. Membrane lipids of Mycoplasma gallisepticum: a disaturated phosphatidylcholine and a phosphatidylglycerol with an unusual positional distribution of fatty acids. Biochemistry. 1979 Jul 10;18(14):2930–2935. doi: 10.1021/bi00581a002. [DOI] [PubMed] [Google Scholar]
- Rottem S. Membrane lipids of mycoplasmas. Biochim Biophys Acta. 1980 May 27;604(1):65–90. doi: 10.1016/0005-2736(80)90585-4. [DOI] [PubMed] [Google Scholar]
- Rottem S., Samuni A. Effect of proteins on the motion of spin-labeled fatty acids in mycoplasma membranes. Biochim Biophys Acta. 1973 Feb 27;298(1):32–38. doi: 10.1016/0005-2736(73)90006-0. [DOI] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- Rottem S., Yashouv J., Ne'eman Z., Razin S. Cholesterol in mycoplasma membranes. Composition, ultrastructure and biological properties of membranes from Mycoplasma mycoides var. capri cells adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):495–508. doi: 10.1016/0005-2736(73)90158-2. [DOI] [PubMed] [Google Scholar]
- Shechter E., Letellier L., Gulik-Krzywicki G. Relations between structure and function in cytoplasmic membrane vesicles isolated from an Escherichia coli fatty-acid auxotroph. High-angle x-ray diffraction, freeze-etch electron microscopy and transport studies. Eur J Biochem. 1974 Nov 1;49(1):61–76. doi: 10.1111/j.1432-1033.1974.tb03811.x. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., de Kruijff B., Gerritsen W. F., Demel R. A., van Deenen L. L., Ververgaert P. H. Freeze-etch electron microscopy of erythrocytes, Acholeplasma laidlawii cells and liposomal membranes after the action of filipin and amphotericin B. Biochim Biophys Acta. 1973 Jan 26;291(2):577–581. doi: 10.1016/0005-2736(73)90509-9. [DOI] [PubMed] [Google Scholar]
- Verkley A. J., Ververgaert P. H., Prins R. A., van Golde L. M. Lipid-phase transitions of the strictly anaerobic bacteria Veillonella parvula and Anaerovibrio lipolytica. J Bacteriol. 1975 Dec;124(3):1522–1528. doi: 10.1128/jb.124.3.1522-1528.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A., Volotovski I. D., Marsh D. Rhodopsin-lipid associations in bovine rod outer segment membranes. Identification of immobilized lipid by spin-labels. Biochemistry. 1979 Oct 30;18(22):5006–5013. doi: 10.1021/bi00589a031. [DOI] [PubMed] [Google Scholar]
- de Kruyff B., de Greef W. J., van Eyk R. V., Demel R. A., van Deenen L. L. The effect of different fatty acid and sterol composition on the erythritol flux through the cell membrane of Acholeplasma laidlawii. Biochim Biophys Acta. 1973 Mar 16;298(2):479–499. doi: 10.1016/0005-2736(73)90375-1. [DOI] [PubMed] [Google Scholar]





