Abstract
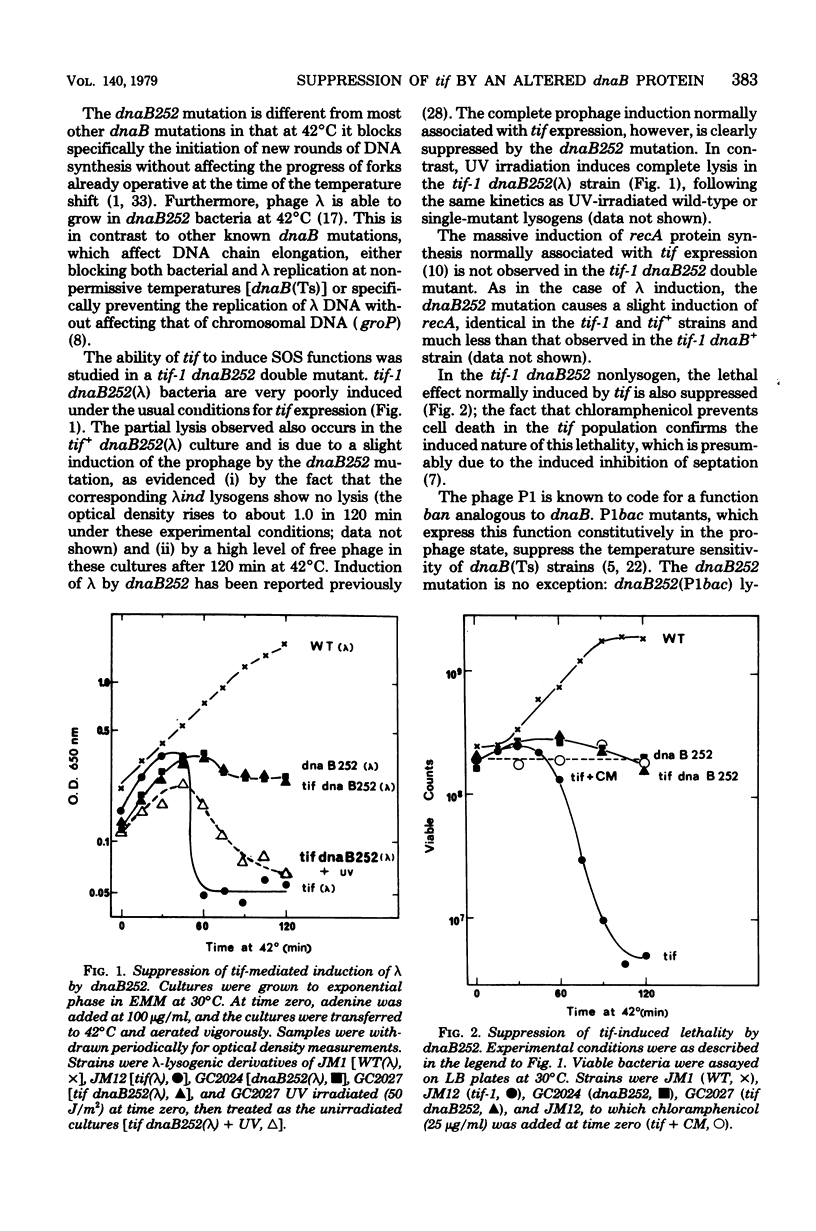
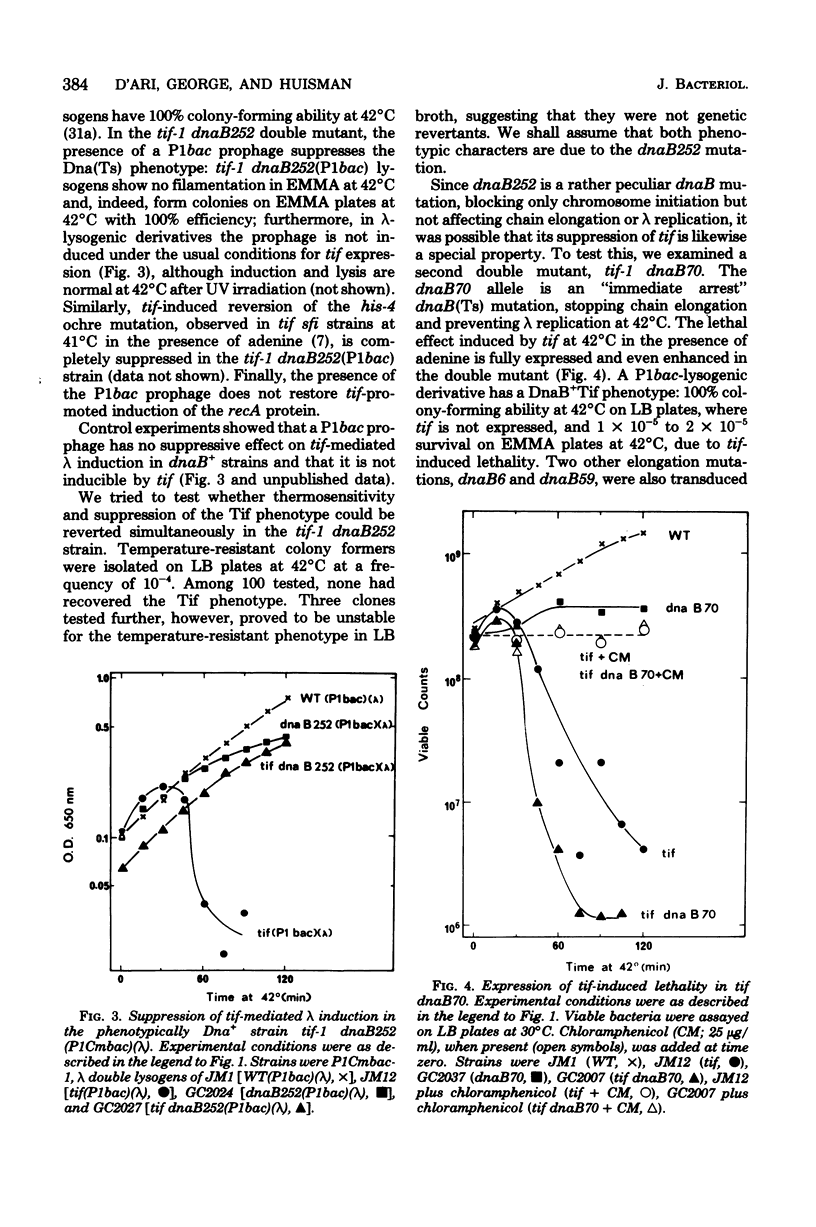
The tif-1 mutation in the Escherichia coli recA gene is known to cause induction of the various "SOS" functions at high temperature, including massive synthesis of the recA protein, lethal filamentation, elevated mutagenesis, and, in lambda lysogens, induction of prophage. It is shown here that the deoxyribonucleic acid initiation mutation dnaB252 suppresses all these manifestations of tif expression. Induction of lambda by ultraviolet irradiation, however, is not affected by the dnaB252 mutation. No similar suppression of tif is observed with other dnaB mutations affecting deoxyribonucleic acid elongation or with other deoxyribonucleic acid initiation mutations at the dnaA and dnaC loci. The fact that an alteration of the dnaB protein specifically suppresses tif-mediated SOS induction implies a role of the replication apparatus in this process, as has been suggested for ultraviolet induction. The induction of lambda is known to proceed via repressor cleavage, presumably promoted by an activated (protease) form of the recA protein. Since lambda induction is normal after ultraviolet irradiation of the tif-1 dnaB252(lambda) strain, tif-mediated induction in this strain may be blocked in a tif-specific step leading to activation of the recA (tif) protein. It is possible that the recA (tif) mutant protein may be directly involved in the replication complex in processes leading to this activation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyersmann D., Schlicht M., Schuster H. Temperature-sensitive initiation of DNA replication in a mutant of Escherichia coli K12. Mol Gen Genet. 1971;111(2):145–158. doi: 10.1007/BF00267789. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., Morand P., George J., Buttin G. Prophage induction and cell division in E. coli. V. Dominance and complementation analysis in partial diploids with pleiotropic mutations (tif, recA, zab and lexB) at the recA locus. Mol Gen Genet. 1977 Jun 24;153(3):297–310. doi: 10.1007/BF00431595. [DOI] [PubMed] [Google Scholar]
- D'Ari R., Jaffé-Brachet A., Touati-Schwartz D., Yarmolinsky M. B. A dnaB analog specified by bacteriophage P1. J Mol Biol. 1975 May 25;94(3):341–366. doi: 10.1016/0022-2836(75)90207-7. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T., West S. C. Identification of protein X of Escherichia coli as the recA+/tif+ gene product. Mol Gen Genet. 1977 Sep 21;155(1):77–85. doi: 10.1007/BF00268563. [DOI] [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Gudas L. J., Mount D. W. Identification of the recA (tif) gene product of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5280–5284. doi: 10.1073/pnas.74.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J. The induction of protein X in DNA repair and cell division mutants of Escherichia coli. J Mol Biol. 1976 Jul 5;104(3):567–587. doi: 10.1016/0022-2836(76)90121-2. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., THERIOT L. A method for selecting radiation-sensitive mutants of Escherichia coli. Genetics. 1962 Sep;47:1219–1224. doi: 10.1093/genetics/47.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Hombrecher G., Vielmetter W. A recA-dependent mutator of Escherichia coli K12: method of isolation and initial characterization. Mutat Res. 1979 Aug;62(1):7–17. doi: 10.1016/0027-5107(79)90218-5. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Maki H., Sekiguchi M. A new conditional lethal mutator (dnaQ49) in Escherichia coli K12. Mol Gen Genet. 1978 Jul 25;163(3):277–283. doi: 10.1007/BF00271956. [DOI] [PubMed] [Google Scholar]
- JACOB F., CAMPBELL A. Sur le système de répression assurant l'immunité chez les bactéries lysogenes. C R Hebd Seances Acad Sci. 1959 Jun 1;248(22):3219–3221. [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- Lanka E., Schuster H. Replication of bacteriophages in Escherichia coli mutants thermosensitive in DNA synthesis. Mol Gen Genet. 1970;106(3):274–285. doi: 10.1007/BF00340386. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Lark C. A. recA-dependent DNA replication in the absence of protein synthesis: characteristics of a dominant lethal replication mutation, dnaT, and requirement for recA+ function. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):537–549. doi: 10.1101/sqb.1979.043.01.059. [DOI] [PubMed] [Google Scholar]
- Maenhaut-Michel G., Brandenburger A., Boiteux S. Requirement of protein and RNA synthesis for lambda repressor inactivation by tif-1: effects of chloramphenicol, neomycin and rifampicin. Mol Gen Genet. 1978 Jul 25;163(3):293–299. doi: 10.1007/BF00271958. [DOI] [PubMed] [Google Scholar]
- McEntee K. Protein X is the product of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5275–5279. doi: 10.1073/pnas.74.12.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMacken R., Ueda K., Kornberg A. Migration of Escherichia coli dnaB protein on the template DNA strand as a mechanism in initiating DNA replication. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4190–4194. doi: 10.1073/pnas.74.10.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. Analysis of dnaB function of Escherichia coli K12 and the dnaB-like function of P1 prophage. J Mol Biol. 1975 May 25;94(3):327–340. doi: 10.1016/0022-2836(75)90206-5. [DOI] [PubMed] [Google Scholar]
- Oishi M., Smith C. L. Inactivation of phage repressor in a permeable cell system: role of recBC DNase in induction. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3569–3573. doi: 10.1073/pnas.75.8.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 1975;5A:355–367. doi: 10.1007/978-1-4684-2895-7_48. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Yoshikawa M. Requirements for suppression of a dnaG mutation by an I-type plasmid. J Bacteriol. 1978 Feb;133(2):485–491. doi: 10.1128/jb.133.2.485-491.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster H., Beyersmann D., Mikolajczyk M., Schlicht M. Prophage induction by high temperature in thermosensitive dna mutants lysogenic for bacteriophage lambda. J Virol. 1973 Jun;11(6):879–885. doi: 10.1128/jvi.11.6.879-885.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R. Genetic studies on bacteriophage P1. Virology. 1968 Dec;36(4):564–574. doi: 10.1016/0042-6822(68)90188-8. [DOI] [PubMed] [Google Scholar]
- Shinagawa H., Mizuuchi K., Emmerson P. T. Induction of prophage lambda by gamma-rays, mitomycin C and tif; repressor cleavage studied by immunoprecipitation. Mol Gen Genet. 1977 Sep 21;155(1):87–91. doi: 10.1007/BF00268564. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Oishi M. Early events and mechanisms in the induction of bacterial SOS functions: analysis of the phage repressor inactivation process in vivo. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1657–1661. doi: 10.1073/pnas.75.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati-Schwartz D. A dnaB analog ban, specified by bacteriophage P1: genetic and physiological evidence for functional analogy and interactions between the two products. Mol Gen Genet. 1979 Jul 13;174(2):173–188. doi: 10.1007/BF00268354. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Smith D. W. NOVEL Escherichia coli dnaB mutant: direct involvement of the dnaB252 gene product in the synthesis of an origin-ribonucleic acid species during initiaion of a round of deoxyribonucleic acid replication. J Bacteriol. 1977 Mar;129(3):1476–1486. doi: 10.1128/jb.129.3.1476-1486.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


