Abstract
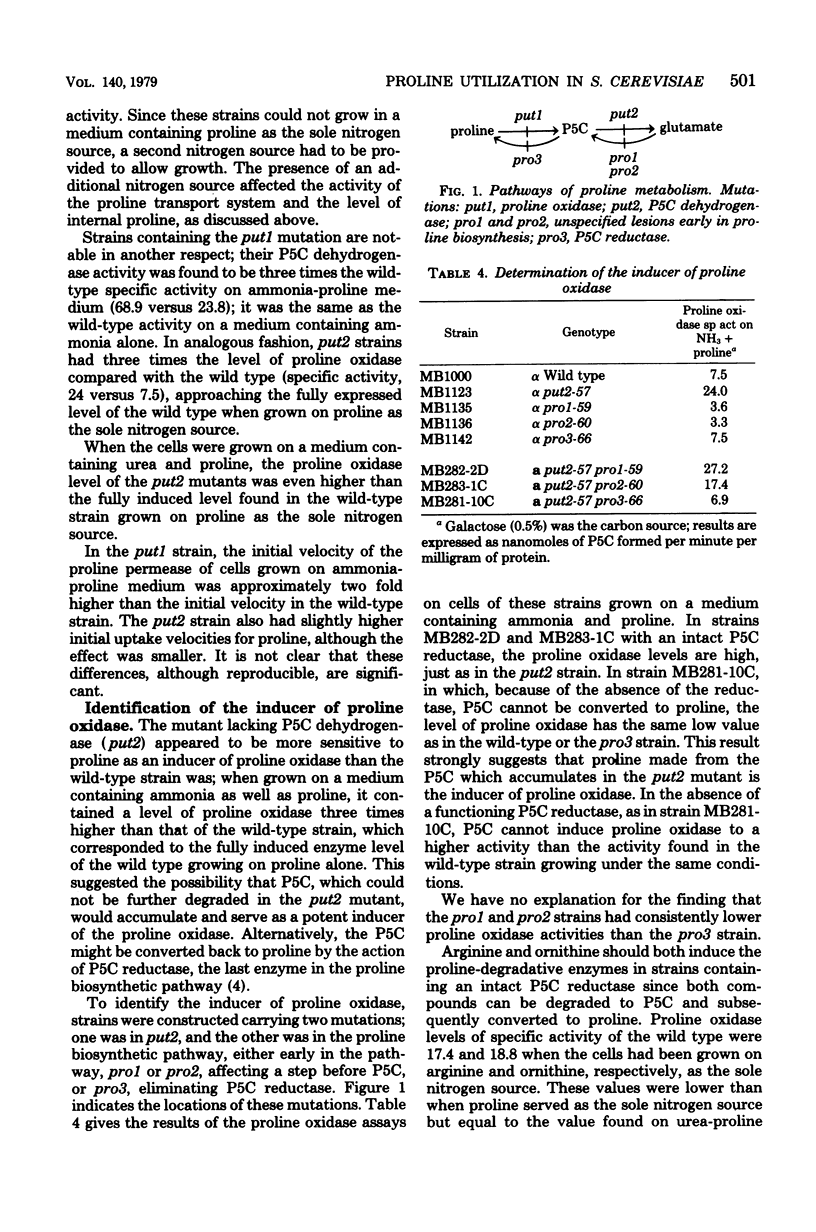
Proline is converted to glutamate in the yeast Saccharomyces cerevisiae by the sequential action of two enzymes, proline oxidase and delta 1-pyrroline-5-carboxylate (P5C) dehydrogenase. The levels of these enzymes appear to be controlled by the amount of proline in the cell. The capacity to transport proline is greatest when the cell is grown on poor nitrogen sources, such as proline or urea. Mutants have been isolated which can no longer utilize proline as the sole source of nitrogen. Mutants in put1 are deficient in proline oxidase, and those in put2 lack P5C dehydrogenase. The put1 and put2 mutations are recessive, segregate 2:2 in tetrads, and appear to be unlinked to one another. Proline induces both proline oxidase and P5C dehydrogenase. The arginine-degradative pathway intersects the proline-degradative pathway at P5C. The P5C formed from the breakdown of arginine or ornithine can induce both proline-degradative enzymes by virtue of its conversion to proline.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arst H. N., Jr, MacDonald D. W. A gene cluster in Aspergillus nidulans with an internally located cis-acting regulatory region. Nature. 1975 Mar 6;254(5495):26–31. doi: 10.1038/254026a0. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, MacDonald D. W. Reduced expression of a distal gene of the prn gene cluster in deletion mutants of Aspergillus nidulans: genetic evidence for a dicistronic messenger in an eukaryote. Mol Gen Genet. 1978 Jul 6;163(1):17–22. doi: 10.1007/BF00268959. [DOI] [PubMed] [Google Scholar]
- Bechet J., Wiame J. M. Indication of a specific regulatory binding protein for ornithinetranscarbamylase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1965 Nov 8;21(3):226–234. doi: 10.1016/0006-291x(65)90276-7. [DOI] [PubMed] [Google Scholar]
- Brandriss M. C. Isolation and preliminary characterization of Saccharomyces cerevisiae proline auxotrophs. J Bacteriol. 1979 Jun;138(3):816–822. doi: 10.1128/jb.138.3.816-822.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandriss M. C., Magasanik B. Genetics and physiology of proline utilization in Saccharomyces cerevisiae: mutation causing constitutive enzyme expression. J Bacteriol. 1979 Nov;140(2):504–507. doi: 10.1128/jb.140.2.504-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendinger S., Brill W. J. Regulation of proline degradation in Salmonella typhimurium. J Bacteriol. 1970 Jul;103(1):144–152. doi: 10.1128/jb.103.1.144-152.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK L., RANHAND B. PROLINE METABOLISM IN ESCHERICHIA COLI. 3. THE PROLINE CATABOLIC PATHWAY. Arch Biochem Biophys. 1964 Aug;107:325–331. doi: 10.1016/0003-9861(64)90338-8. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundgren D. W., Ogur M., Yuen S. The isolation and characterization of a Saccharomyces mutant deficient in 1 -pyrroline- 5 -carboxylate dehydrogenase activity. Biochim Biophys Acta. 1972 Dec 29;286(2):360–362. doi: 10.1016/0304-4165(72)90271-1. [DOI] [PubMed] [Google Scholar]
- MIDDELHOVEN W. J. THE PATHWAY OF ARGININE BREAKDOWN IN SACCHAROMYCES CEREVISIAE. Biochim Biophys Acta. 1964 Dec 9;93:650–652. doi: 10.1016/0304-4165(64)90349-6. [DOI] [PubMed] [Google Scholar]
- Middelhoven W. J., Broekhuizen B., van Eijk J. Detection, with the dye phloxine B, of yeast mutants unable to utilize nitrogenous substances as the sole nitrogen source. J Bacteriol. 1976 Dec;128(3):851–852. doi: 10.1128/jb.128.3.851-852.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzkin B., Roth J. Cluster of genes controlling proline degradation in Salmonella typhimurium. J Bacteriol. 1978 Feb;133(2):744–754. doi: 10.1128/jb.133.2.744-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwencke J., Magaña-Schwencke N. Derepression of a proline transport system in Saccharomyces chevalieri by nitrogen starvation. Biochim Biophys Acta. 1969 Mar 11;173(2):302–312. doi: 10.1016/0005-2736(69)90113-8. [DOI] [PubMed] [Google Scholar]
- Stewart P. R. Analytical methods for yeasts. Methods Cell Biol. 1975;12:111–147. doi: 10.1016/s0091-679x(08)60955-3. [DOI] [PubMed] [Google Scholar]
- Williams I., Frank L. Improved chemical synthesis and enzymatic assay of delta-1-pyrroline-5-carboxylic acid. Anal Biochem. 1975 Mar;64(1):85–97. doi: 10.1016/0003-2697(75)90408-x. [DOI] [PubMed] [Google Scholar]


