Abstract
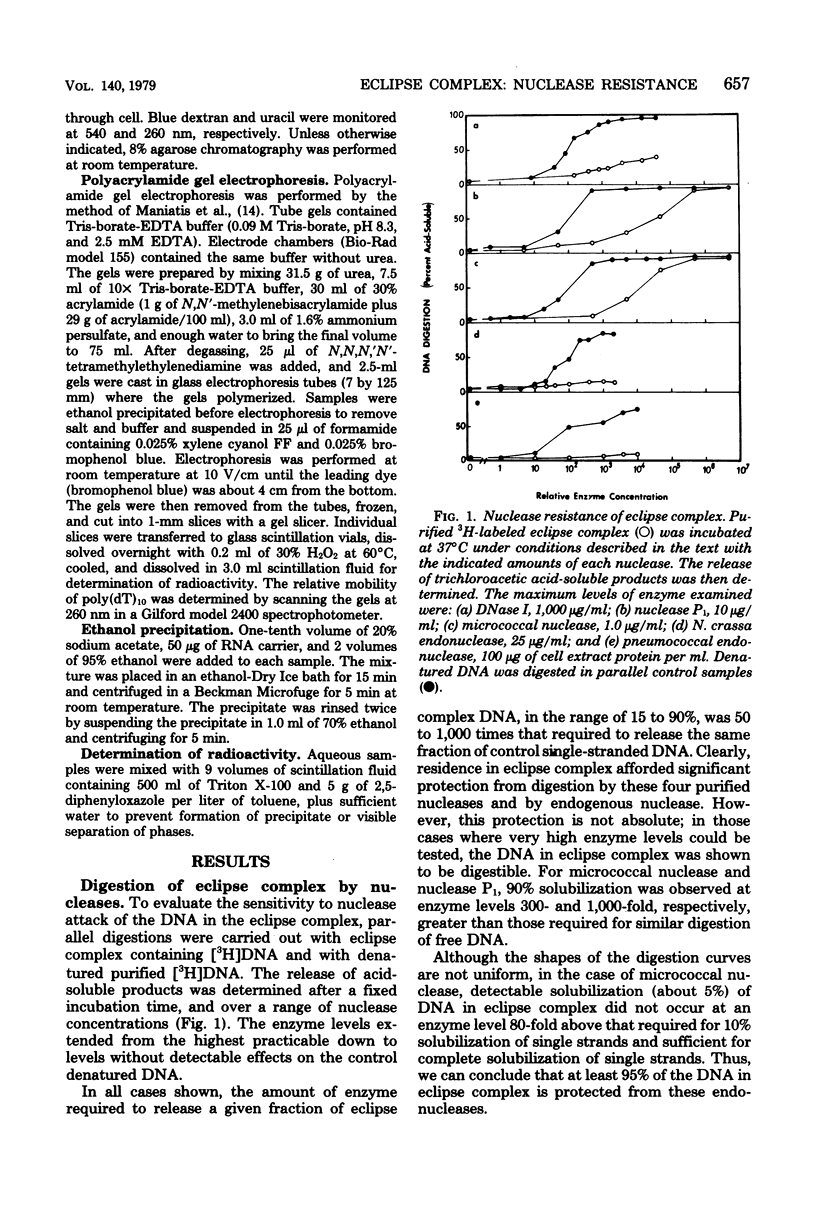
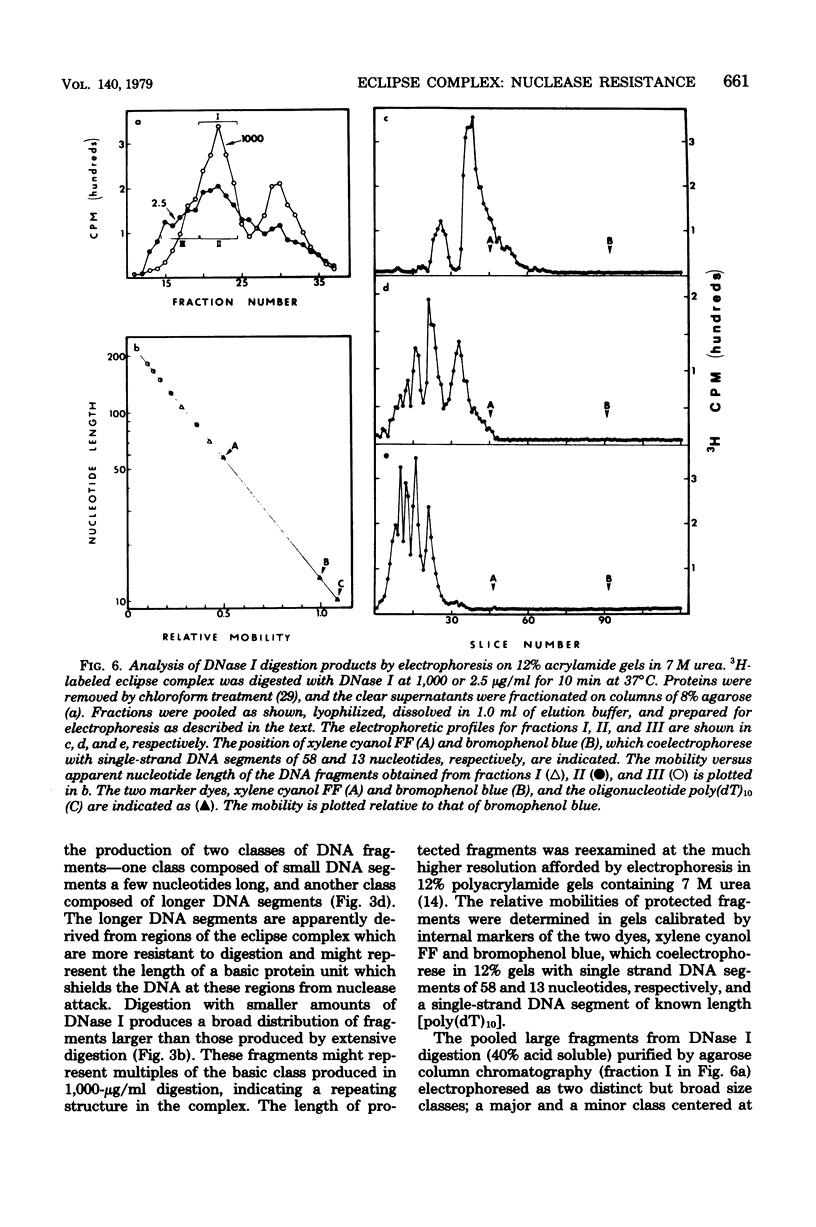
Donor deoxyribonucleic acid strands in the eclipse phase of genetic transformation of pnuemococcus (Streptococcus pneumoniae) are purified as a complex with a cf the deoxyribonucleic acid strand in this complex to digestion by nucleases was shown to be 50- to 1,000-fold less than that of uncomplexed single strands of deoxyribonucleic acid. Deoxyribonuclease I, micrococcal nuclease, Neurospora endonuclease, nuclease P1, and the major endogenous nuclease of cell-free extracts were studied. Sensitivity to nuclease attack was not uniform along the deoxyribonucleic acid strand; sequences of strongly protected bases were separated by more sensitive regions. The minimum size of protected fragments was about 70 bases. A complex of protein with the protected deoxyribonucleic acid segments was obtained after partial digestion. The sizes of these complexes, of the protected deoxyribonucleic acid segments, and of the protein subunit released by complete nuclease digestion, are all approximately identical, as determined by gel exclusion chromatography. Deoxyribonucleic acid strands of eclipse complex were also shown to be particularly well protected from attack by the major pneumococcal endonuclease in cell extracts.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins C. J., Guild W. R. Events occurring near the time of synapsis during transformation in Diplococcus pneumoniae. J Bacteriol. 1972 Jan;109(1):266–275. doi: 10.1128/jb.109.1.266-275.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt E., Lange R., Willecke K. Competent Bacillus subtilis cultures synthesize a denatured DNA binding activity. Proc Natl Acad Sci U S A. 1975 Jan;72(1):323–327. doi: 10.1073/pnas.72.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghei O. K., Lacks S. A. Recovery of donor deoxyribonucleic acid marker activity from eclipse in pneumococcal transformation. J Bacteriol. 1967 Mar;93(3):816–829. doi: 10.1128/jb.93.3.816-829.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney T., Jr, Fox M. S. Physical and genetic hybrids formed in bacterial transformation. J Mol Biol. 1968 Feb 28;32(1):83–100. doi: 10.1016/0022-2836(68)90147-2. [DOI] [PubMed] [Google Scholar]
- Huang W. M., Lehman I. R. On the exonuclease activity of phage T4 deoxyribonucleic acid polymerase. J Biol Chem. 1972 May 25;247(10):3139–3146. [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Deoxyribonucleases of Pneumococcus. J Biol Chem. 1967 Jul 10;242(13):3108–3120. [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Identification of a deoxyribonuclease implicated in genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1975 Jul;123(1):222–232. doi: 10.1128/jb.123.1.222-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Single-strand breakage on binding of DNA to cells in the genetic transformation of Diplococcus pneumoniae. J Mol Biol. 1976 Feb 25;101(2):255–275. doi: 10.1016/0022-2836(76)90376-4. [DOI] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Neuberger M. Membrane location of a deoxyribonuclease implicated in the genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1975 Dec;124(3):1321–1329. doi: 10.1128/jb.124.3.1321-1329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay V., Linn S. Selective inhibition of the dnase activity of the recBC enzyme by the DNA binding protein from Escherichia coli. J Biol Chem. 1976 Jun 25;251(12):3716–3719. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Molineux I. J., Friedman S., Gefter M. L. Purification and properties of the Escherichia coli deoxyribonucleic acid-unwinding protein. Effects on deoxyribonucleic acid synthesis in vitro. J Biol Chem. 1974 Oct 10;249(19):6090–6098. [PubMed] [Google Scholar]
- Molineux I. J., Gefter M. L. Properties of the Escherichia coli DNA-binding (unwinding) protein interaction with nucleolytic enzymes and DNA. J Mol Biol. 1975 Nov 15;98(4):811–825. doi: 10.1016/s0022-2836(75)80012-x. [DOI] [PubMed] [Google Scholar]
- Molineux I. J., Gefter M. L. Properties of the Escherichia coli in DNA binding (unwinding) protein: interaction with DNA polymerase and DNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3858–3862. doi: 10.1073/pnas.71.10.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Breakage prior to entry of donor DNA in Pneumococcus transformation. Biochim Biophys Acta. 1973 Apr 11;299(4):545–556. doi: 10.1016/0005-2787(73)90226-8. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Transformation and deoxyribonucleic acid size: extent of degradation on entry varies with size of donor. J Bacteriol. 1972 Dec;112(3):1157–1168. doi: 10.1128/jb.112.3.1157-1168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in pneumococcus: existence and properties of a complex involving donor deoxyribonucleate single strands in eclipse. J Bacteriol. 1977 Nov;132(2):576–583. doi: 10.1128/jb.132.2.576-583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in pneumococcus: protein content of eclipse complex. J Bacteriol. 1978 Nov;136(2):548–557. doi: 10.1128/jb.136.2.548-557.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass K., Frenkel G. D. Adenovirus-induced inhibition of cellular DNase. J Virol. 1978 May;26(2):540–543. doi: 10.1128/jvi.26.2.540-543.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oey J. L., Knippers R. Properties of the isolated gene 5 protein of bacteriophage fd. J Mol Biol. 1972 Jul 14;68(1):125–138. doi: 10.1016/0022-2836(72)90268-9. [DOI] [PubMed] [Google Scholar]
- Pacumbaba R., Center M. S. Partial purification and properties of a bacteriophage T7 inhibitor of the host exonuclease V activity. J Virol. 1975 Nov;16(5):1200–1207. doi: 10.1128/jvi.16.5.1200-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieniazek D., Piechowska M., Venema G. Characteristics of a complex formed by a nonintegrated fraction of transforming DNA and Bacillus subtilis recipient cell constituents. Mol Gen Genet. 1977 Nov 18;156(3):251–261. doi: 10.1007/BF00267179. [DOI] [PubMed] [Google Scholar]
- Raina J. L., Ravin A. W. Fate of homospecific transforming DNA bound to Streptococcus sanguis. J Bacteriol. 1978 Mar;133(3):1212–1223. doi: 10.1128/jb.133.3.1212-1223.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Guild W. R. Kinetics of integration of transforming DNA in pneumococcus. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3331–3335. doi: 10.1073/pnas.69.11.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]


