Abstract
We have examined the accumulation of polyphosphorylated nucleotides in Bacillus subtilis in relation to the function of the rel gene. Our results are as follows. (i) During inhibition of isoleucine activation by O-methylthreonine, wildtype B. subtilis cells accumulate unusual nucleotides with the chromatographic and chemical properties of pppApp, ppApp, pppGpp, ppGpp, pGpp, and ppGp. (ii) During the carbon source downshift elicited by inhibiting glucose uptake, we observed accumulation of the polyphosphorylated guanosine but not adenosine nucleotides. (iii) At the end of long phase in sporulation medium, we observed a small transient accumulation of the polyphosphorylated guanosine but not adenosine nucleotides. (iv) We were unable to detect a nucleotide with chromatographic behavior expected for pppAppp under any conditions. (v) The rel mutant of Swanton and Edlin (Biochem. Biophys. Res. Commun. 46-583-588, 1972) did not accumulate any of these polyphosphorylated nucleotides under any of the conditions examined. (vi) the rel mutant is unimpaired in sporulation. We conclude that one or more of the nucleotides we have detected may be involved in controlling the specificity of transcription during the stringent response, but none of them are required for sporogenesis.
Full text
PDF






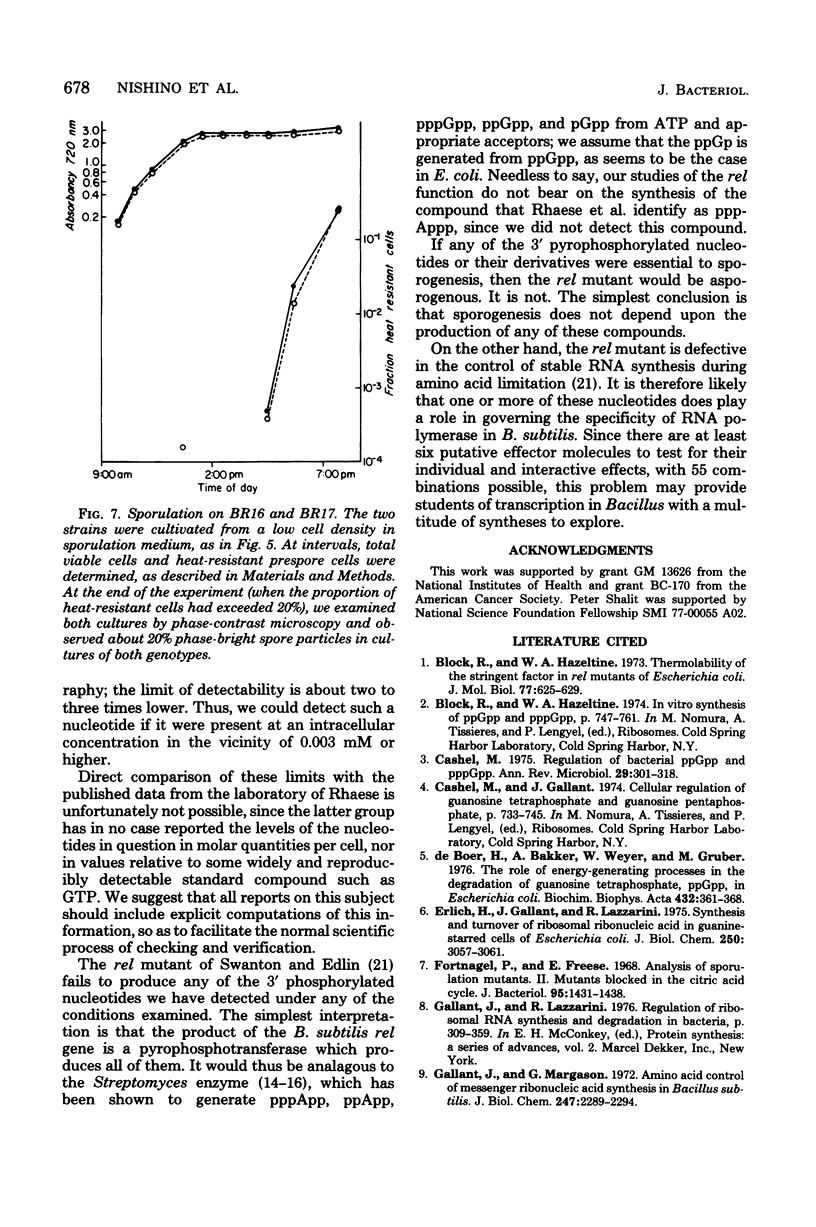

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block R., Haseltine W. A. Thermolability of the stringent factor in rel mutants of Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):625–629. doi: 10.1016/0022-2836(73)90228-3. [DOI] [PubMed] [Google Scholar]
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- De Boer H. A., Bakker A. J., Weyer W. J., Gruber M. The role of energy-generating processes in the degradation of guanosine tetrophosphate, ppGpp, in Escherichia coli. Biochim Biophys Acta. 1976 May 19;432(3):361–368. doi: 10.1016/0005-2787(76)90146-5. [DOI] [PubMed] [Google Scholar]
- Erlich H., Gallant J. Synthesis and turnover of ribosomal ribonucleic acid in guanine-starved cells of Escherichia coli. J Biol Chem. 1975 Apr 25;250(8):3057–3061. [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Margason G. Amino acid control of messenger ribonucleic acid synthesis in Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2289–2294. [PubMed] [Google Scholar]
- Gallant J., Margason G., Finch B. On the turnover of ppGpp in Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6055–6058. [PubMed] [Google Scholar]
- Hansen M. T., Pato M. L., Molin S., Fill N. P., von Meyenburg K. Simple downshift and resulting lack of correlation between ppGpp pool size and ribonucleic acid accumulation. J Bacteriol. 1975 May;122(2):585–591. doi: 10.1128/jb.122.2.585-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Mukai J., Kukita T., Murao S., Nishino T. Acceptor specificity of ATP:nucleoside-5'-phosphate pyrophosphotransferase from Streptomyces adephospholyticus. Synthesis of the 3'-pyrophosphates of pyrimidine nucleotides, some oligoribonucleotides, 5'-diphosphonucleosidic coenzymes and mG-5'-ppp-5'-Am. J Biochem. 1978 Apr;83(4):1209–1212. doi: 10.1093/oxfordjournals.jbchem.a132014. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Gallant J. A new nucleotide involved in the stringent response in Escherichia coli. Guanosine 5'-diphosphate-3'-monophosphate. J Biol Chem. 1979 Feb 10;254(3):688–692. [PubMed] [Google Scholar]
- Rhaese H. J., Grade R., Dichtelmüller H. Studies on the control of development. Correlation of initiucleotides in Bacillus subtilis. Eur J Biochem. 1976 Apr 15;64(1):205–213. doi: 10.1111/j.1432-1033.1976.tb10289.x. [DOI] [PubMed] [Google Scholar]
- Rhaese H. J., Groscurth R. Control of development: role of regulatory nucleotides synthesized by membranes of Bacillus subtilis in initiation of sporulation. Proc Natl Acad Sci U S A. 1976 Feb;73(2):331–335. doi: 10.1073/pnas.73.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaese H. J., Groscurth R. Studies on the control of development. Synthesis of two highly phosphorylated nucleotides depends on changes in the composition of ribosomes at the beginning of sporulation in Bacillus subtilis. Eur J Biochem. 1978 Apr 17;85(2):517–528. doi: 10.1111/j.1432-1033.1978.tb12267.x. [DOI] [PubMed] [Google Scholar]
- Swanton M., Edlin G. Isolation and characterization of an RNA relaxed mutant of B. subtilis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):583–588. doi: 10.1016/s0006-291x(72)80179-7. [DOI] [PubMed] [Google Scholar]






