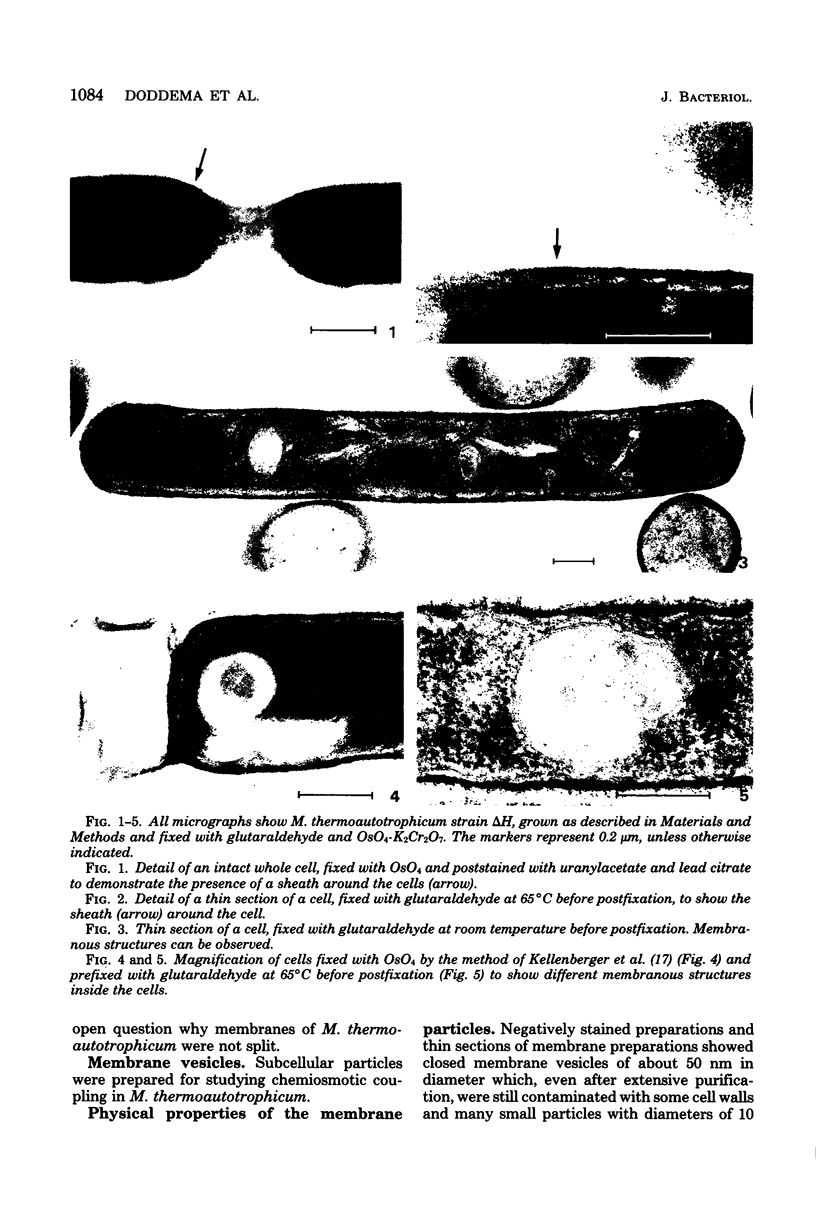
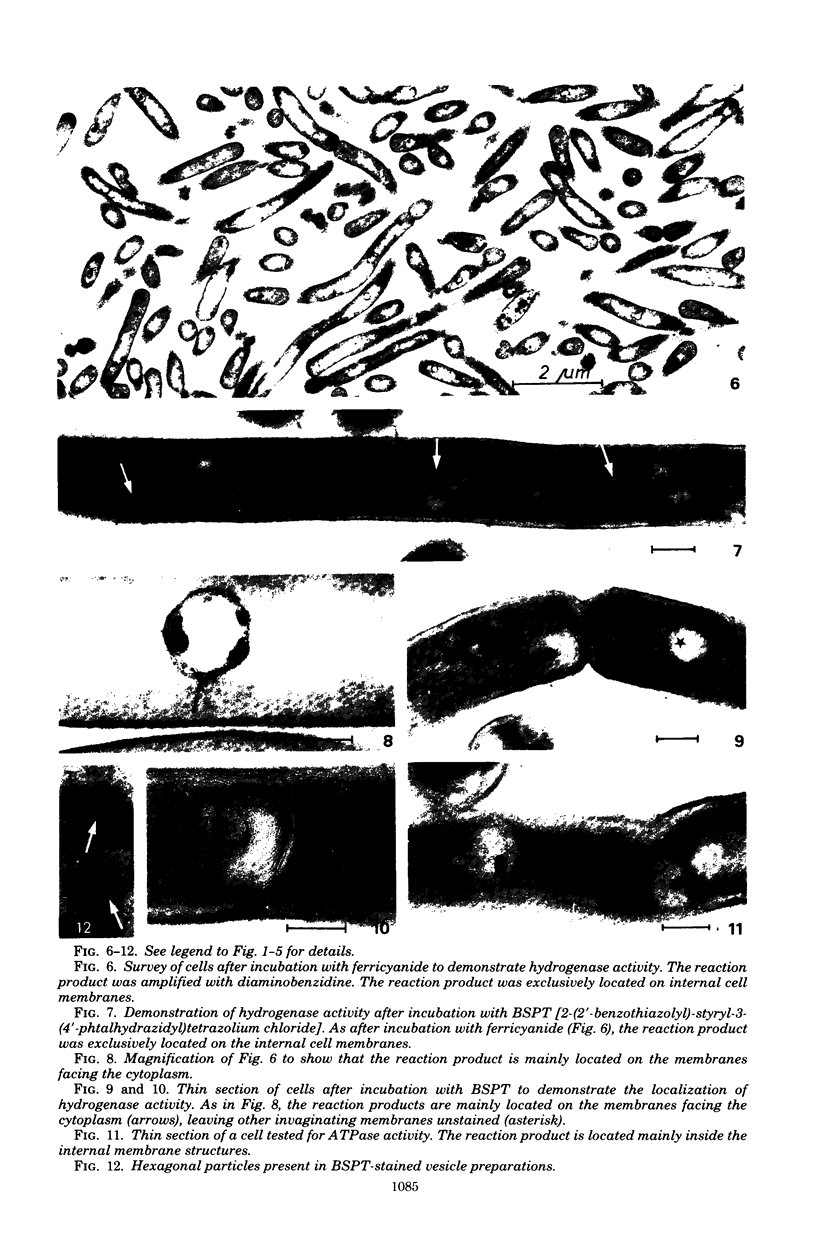
Abstract
Hydrogenase and the adenosine 5'-triphosphate (ATP) synthetase complex, two enzymes essential in ATP generation in Methanobacterium thermoautotrophicum, were localized in internal membrane systems as shown by cytochemical techniques. Membrane vesicles from this organism possessed hydrogenase and adenosine triphosphatase (ATPase) activity and synthesized ATP driven by hydrogen oxidation or a potassium gradient. ATP synthesis depended on anaerobic conditions and could be inhibited in membrane vesicles by uncouplers, nigericin, or the ATPase inhibitor N,N'-dicyclohexylcarbodiimide. The presence of an adenosine 5'-diphosphate-ATP translocase was postulated. With fluorescent dyes, a membrane potential and pH gradient were demonstrated.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird C. W., Lynch J. M., Pirt F. J., Reid W. W. Steroids and squalene in Methylococcus capsulatus grown on methane. Nature. 1971 Apr 16;230(5294):473–474. doi: 10.1038/230473a0. [DOI] [PubMed] [Google Scholar]
- CRANE F. L., DILLEY R. A. DETERMINATION OF COENZYME Q (UBIQUINONE). Methods Biochem Anal. 1963;11:279–306. doi: 10.1002/9780470110294.ch6. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. L., Whittenbury R. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- De Souza N. J., Nes W. R. Sterols: isolation from a blue-green alga. Science. 1968 Oct 18;162(3851):363–363. doi: 10.1126/science.162.3851.363. [DOI] [PubMed] [Google Scholar]
- Doddema H. J., Hutten T. J., van der Drift C., Vogels G. D. ATP hydrolysis and synthesis by the membrane-bound ATP synthetase complex of Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Oct;136(1):19–23. doi: 10.1128/jb.136.1.19-23.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddema H. J., Vogels G. D. Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1978 Nov;36(5):752–754. doi: 10.1128/aem.36.5.752-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilermann L. J., Pandit-Hovenkamp H. G., Kolk A. H. Oxidative phosphorylation in Azotobacter vinelandii particles. Phosphorylation sites and respiratory control. Biochim Biophys Acta. 1970 Jan 13;197(1):25–30. doi: 10.1016/0005-2728(70)90004-6. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Shechter I. Occurrence of squalene in methanol-grown bacteria. J Bacteriol. 1978 Aug;135(2):717–720. doi: 10.1128/jb.135.2.717-720.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenawalt J. W., Whiteside T. L. Mesosomes: membranous bacterial organelles. Bacteriol Rev. 1975 Dec;39(4):405–463. doi: 10.1128/br.39.4.405-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman A., Bittan R., Carmeli C. Nucleotide translocation across the cytoplasmic membrane in the photosynthetic bacterium Rhodopseudomonas capsulata. FEBS Lett. 1978 May 1;89(1):21–25. doi: 10.1016/0014-5793(78)80513-4. [DOI] [PubMed] [Google Scholar]
- Hwang S. B., Stoeckenius W. Purple membrane vesicles: morphology and proton translocation. J Membr Biol. 1977 May 12;33(3-4):325–350. doi: 10.1007/BF01869523. [DOI] [PubMed] [Google Scholar]
- Ishaque M., Aleem M. I. Energy coupling in Hydrogenomonas eutropha. Biochim Biophys Acta. 1970 Dec 8;223(2):388–397. doi: 10.1016/0005-2728(70)90196-9. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O., König H. Chemical composition of the peptidoglycan-free cell walls of methanogenic bacteria. Arch Microbiol. 1978 Aug 1;118(2):141–152. doi: 10.1007/BF00415722. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langenberg K. F., Bryant M. P., Wolfe R. S. Hydrogen-oxidizing methane bacteria. II. Electron microscopy. J Bacteriol. 1968 Mar;95(3):1124–1129. doi: 10.1128/jb.95.3.1124-1129.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K., Hilliker K. Passive potassium ion permeability of Halobacterium halobium cell envelope membranes. Biochim Biophys Acta. 1976 Sep 21;448(1):181–184. doi: 10.1016/0005-2736(76)90086-9. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Light energy conversion in Halobacterium halobium. Microbiol Rev. 1978 Dec;42(4):682–706. doi: 10.1128/mr.42.4.682-706.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOOR H. DIE GEFRIER-FIXATION LEBENDER ZELLEN UND IHRE ANWENDUNG IN DER ELEKTRONENMIKROSKOPIE. Z Zellforsch Mikrosk Anat. 1964 Apr 28;62:546–580. [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R. A., Singer M. E. Ether-containing lipids of methanogenic bacteria. Biochem Biophys Res Commun. 1978 May 30;82(2):716–722. doi: 10.1016/0006-291x(78)90933-6. [DOI] [PubMed] [Google Scholar]
- Mayer F., Lurz R., Schoberth S. Electron microscopic investigation of the hydrogen-oxidizing acetate-forming anaerobic bacterium Acetobacterium woodii. Arch Microbiol. 1977 Nov 18;115(2):207–213. doi: 10.1007/BF00406376. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Rechardt L., Kokko A. Electron microscopic observations on the mitochondrial adenosinetriphosphatase in the rat spinal cord. Histochemie. 1967;10(3):278–286. doi: 10.1007/BF00304876. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Wolfe R. S. Adenosine triphosphate pools in Methanobacterium. J Bacteriol. 1970 Apr;102(1):43–51. doi: 10.1128/jb.102.1.43-51.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitka T. K., Talibi G. G. Cytochemical localization by ferricyanide reduction of -hydroxy acid oxidase activity in peroxisomes of rat kidney. Histochemie. 1971;27(2):137–158. doi: 10.1007/BF00284956. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene T. G., Kates M., Gelpi E., Oro J. Occurrence of squalene, di- and tetrahydrosqualenes, and vitamin MK8 in an extremely halophilic bacterium, Halobacterium cutirubrun. J Lipid Res. 1969 May;10(3):294–303. [PubMed] [Google Scholar]
- Tornabene T. G., Wolfe R. S., Balch W. E., Holzer G., Fox G. E., Oro J. Phytanyl-glycerol ethers and squalenes in the archaebacterium Methanobacterium thermoautotrophicum. J Mol Evol. 1978 Aug 2;11(3):259–266. doi: 10.1007/BF01734487. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. Adenosine 5'-triphosphate synthesis energized by an artificially imposed membrane potential in membrane vesicles of Escherichia coli. J Bacteriol. 1976 Jul;127(1):154–161. doi: 10.1128/jb.127.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner A. Optical probes of membrane potential. J Membr Biol. 1976 Jun 30;27(4):317–334. doi: 10.1007/BF01869143. [DOI] [PubMed] [Google Scholar]
- Weerkamp A., Heinen-von Borries U. T., Vogels G. D. Biochemical and ultrastructural changes in Staphylococcus aureus treated with staphylococcin 1580. Antonie Van Leeuwenhoek. 1978;44(1):35–48. doi: 10.1007/BF00400075. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Fox G. E. Archaebacteria. J Mol Evol. 1978 Aug 2;11(3):245–251. doi: 10.1007/BF01734485. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Fine structure of Methanobacterium thermoautotrophicum: effect of growth temperature on morphology and ultrastructure. J Bacteriol. 1973 Jan;113(1):461–467. doi: 10.1128/jb.113.1.461-467.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]