Abstract
This study was conducted to examine the rate of contamination and the molecular characteristics of enteric bacteria isolated from a selection of food sources in Vietnam. One hundred eighty raw food samples were tested; 60.8% of meat samples and 18.0% of shellfish samples were contaminated with Salmonella spp., and more than 90% of all food sources contained Escherichia coli. The isolates were screened for antibiotic resistance against 15 antibiotics, and 50.5% of Salmonella isolates and 83.8% of E. coli isolates were resistant to at least one antibiotic. Isolates were examined for the presence of mobile genetic elements conferring antibiotic resistance. Fifty-seven percent of E. coli and 13% of Salmonella isolates were found to contain integrons, and some isolates contained two integrons. Sequencing results revealed that the integrons harbored various gene cassettes, including aadA1, aadA2, and aadA5 (resistance to streptomycin and spectinomycin), aacA4 (resistance to aminoglycosides), the dihydrofolate reductase gene cassettes dhfrXII, dfrA1, and dhfrA17 (trimethoprim resistance), the beta-lactamase gene blaPSE1 (ampicillin resistance), and catB3 (chloramphenicol resistance). Plasmids were also detected in all 23 antibiotic-resistant Salmonella isolates and in 33 E. coli isolates. Thirty-five percent of the Salmonella isolates and 76% of the E. coli isolates contained plasmids of more than 95 kb, and some of the isolates contained two large plasmids. Conjugation experiments showed the successful transfer of all or part of the antibiotic resistance phenotypes among the Salmonella and E. coli food isolates. Our results show that enteric bacteria in raw food samples from Vietnam contain a pool of mobile genetic elements and that the transfer of antibiotic resistance can readily occur between similar bacteria.
Food contamination with antibiotic-resistant bacteria can be a major threat to public health, as the antibiotic resistance determinants can be transferred to other bacteria of human clinical significance. The prevalence of antimicrobial resistance among food-borne pathogens has increased during recent decades (9, 15, 18, 56), possibly as the result of selection pressure created by the use of antimicrobials in food-producing animals (1, 4, 11, 55, 59). The coexistence of resistance genes with mobile elements such as plasmids, transposons, and integrons facilitates the rapid spread of antibiotic resistance genes among bacteria (53). Molecular analysis of antibiotic resistance genes and antibiotic-resistant mobile elements has shown that identical elements were found in bacteria that colonize both animals and humans, suggesting a role for raw foods in the dissemination of resistant bacteria and resistance genes to humans via the food chain (33, 44, 55).
Information on the phenotypes and genotypes of antimicrobial resistance in food-borne microorganisms is largely restricted to first-world countries, and there is a paucity of information on what is happening in developing countries. Where they are reported, rates of resistance to antibiotics of bacteria originating from meat were high in developing countries (2, 3, 16, 37, 54), possibly as the result of the inappropriate or uncontrolled use of antibiotics in farming practices. Therefore, the study of antibiotic resistance in developing countries is important as the information could enhance prudent use of antibiotics in food production. In Vietnam, antibiotic resistance has been reported to occur in human bacterial isolates, including Salmonella enterica serovar Typhi and other diarrhea-causing pathogens (5, 12, 22, 26, 40). However, as far as we are aware, there has been very little published about the occurrence of antibiotic-resistant bacteria in raw food samples in Vietnam and even less about the molecular characteristics of these antibiotic-resistant bacteria. This study was conducted to address some of these issues and to provide a current baseline of information on molecular characteristics of antibiotic resistance of Salmonella and Escherichia coli isolates from foods commonly sold in the marketplace in Vietnam. The isolates were investigated for the presence of class 1 integrons and their associated gene cassettes and for the presence of plasmids. The transferability of antibiotic resistance was examined by conjugation.
MATERIALS AND METHODS
Isolation and identification of E. coli and Salmonella spp.
One hundred eighty samples of meat, consisting of beef (n = 50 samples), chicken/poultry (n = 30 samples), pork (n = 50 samples), and shellfish (n = 50 samples), were purchased from various markets and supermarkets around Ho Chi Minh City between February and June 2004 for the isolation and identification of Salmonella spp. and E. coli. Forty-three samples from chicken feces were also collected for E. coli isolation from chickens less than 1 month old. The procedures for the isolation of Salmonella spp. and E. coli were based on the Nordic Committee on Food Analysis methods (41, 42). Salmonella isolates were further grouped with commercial monovalent sera (Remel, Inc.), used according to the manufacturer's instructions. The representative salmonella isolates were further serotyped by the Microbiological Diagnostic Unit, Melbourne University, Australia.
Antibiotic susceptibility tests.
Antibiotic resistance of E. coli and Salmonella isolates was determined by the disk diffusion method using the standard procedure of the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) (39). The isolates were classified as susceptible, intermediate, or resistant according to interpretation of the zone diameter standards recommended by CLSI (17). The concentration of the discs (Oxoid, Australia) and the abbreviations of antimicrobial agents used throughout this report are ampicillin (AMP), 10 μg; amoxicillin (AMX), 10 μg; amoxicillin-clavulanic acid (AMC), 30 μg; cephalothin (CEF), 30 μg; chloramphenicol (CHL), 30 μg; ciprofloxacin (CIP), 5 μg; enrofloxacin (ENR), 5 μg; tetracycline (TET), 30 μg; gentamicin (GEN), 10 μg; kanamycin (KAN), 30 μg; nalidixic acid (NAL), 30 μg; norfloxacin (NOR), 10 μg; sulfafurazole (SUL), 300 μg; streptomycin (STR), 10 μg; and trimethoprim (TMP), 5 μg. The reference strains Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used to verify the quality and accuracy of the testing procedures.
Detection of class 1 integrons.
Twenty-three antibiotic-resistant Salmonella isolates and 35 E. coli isolates, which showed the highest degree of resistance among the collection, were examined for the presence of class 1 integrons by PCR using primers and conditions as described previously (36). PCRs were carried out in a total volume 50 μl containing 2 μl of boiled bacterial suspension, each deoxyribonucleotide at a concentration of 0.25 mM, 2 mM MgCl2, 1 U of AmpliTaq Gold DNA polymerase (Roche, Germany), and 0.4 μM of each primer. Thermal cycler reaction conditions included an initial denaturation at 95°C for 5 min, followed by 35 cycles, each of which consisted of denaturation at 95°C for 30 s, annealing time at 56°C for 30 s, and a final 7-min extension at 72°C. Salmonella enterica serovar Typhimurium strain DT104, which contained 1.0-kb and 1.2-kb integrons, was used as a positive control. PCR products were digested with at least three separate restriction endonucleases. Isolates with the same PCR amplicon sizes and identical restriction patterns were randomly chosen for complete sequence analyses using an ABI Prism BigDye Terminator cycle sequencing Ready Reaction kit (Perkin-Elmer Corp.) according to the manufacturer's protocol and were sequenced at Monash University, Victoria, Australia. The sequences obtained were analyzed using BLAST (http://www.ncbi.nih.gov) and compared with those registered in GenBank.
Plasmid extraction.
The plasmid extraction method used was based on the Kado and Liu method, with some modifications (28). Strains were grown in 10 ml of Luria-Bertani (LB) broth containing appropriate antibiotic at 37°C with shaking to exponential stage. Cells from 1.5 ml of culture were harvested, and the pellet was resuspended in 200 μl of Tris-EDTA buffer. The cells were lysed by the addition of 400 μl of lysis solution (containing 3% [wt/vol] sodium dodecyl sulfate and 50 mM Tris [pH 12.6]). The mixture was incubated at 60°C for 1 h. Proteins and chromosomal DNA were then precipitated by the addition of 900 μl of 1:1 (vol/vol) phenol-chloroform, and the precipitate was removed by centrifugation. The aqueous DNA solution was then freed of phenol by extraction with 1 volume of chloroform. The upper aqueous layer containing the plasmid DNA was collected and was either loaded on a gel for electrophoresis or stored at −20°C. The gel was prepared at 0.7% in 1× Tris-acetate-EDTA buffer, and electrophoresis was carried out at 70 V for 2.5 h. Salmonella serovar Typhimurium strain 82/6915, which contained a single 95-kb plasmid, was used as a control. Sizes of the plasmids were compared using a BAC-Tracker supercoiled ladder, with sizes ranging from 8 to 165 kb (Epicenter). Isolates used for integron detection (except for isolates E/C/15a and E/SF/29) were evaluated for the presence of plasmids.
Conjugation study.
Spread-plate mating and liquid mating methods (30) were used in the conjugation experiments with different donor-recipient combinations. Selected strains which contained plasmids of more than 95 kb were used, including S/C/5 (Salmonella serovar Havana, chicken isolate), S/C/9b (Salmonella serovar Havana, chicken isolate), S/P/24 (Salmonella serovar Anatum, pork isolate), E/C/4a (E. coli, chicken isolate), and E/C/5a (E. coli, chicken isolate). Recipient laboratory strains and strains isolated for this study included E/P/8a (E. coli, pork isolate), E/F/13 (E. coli, chicken feces isolate), E/F/16 (E. coli, chicken feces isolate), E. coli HB101 (E. coli, laboratory strain), E. coli JM109 (E. coli, laboratory strain), S/P/9 (Salmonella serovar Typhimurium, pork isolate), and S/P/13 (Salmonella serovar Anatum, pork isolate). For the spread-plate mating, the donor and recipient strains were grown separately overnight with appropriate antibiotics, and then 100 μl of donor and 100 μl of recipient strains at different concentrations were spread plated onto LB agar plates containing both of the selected antibiotics. In liquid mating, 0.5 ml and 1.0 ml of overnight-incubated donor and recipient broth cultures, respectively, were mixed in 10 ml of LB broth. The mixtures were then incubated overnight without shaking. Then, 0.2-ml volumes of each mixture at different concentration were spread onto LB-agar plates containing both of the selected antibiotics. Colonies from the selector plates were picked off and identified again after plates were incubated at 37°C for 24 h and their antibiotic resistance phenotypes were determined.
RESULTS
Presence of class 1 integrons and resistance gene cassettes in Salmonella spp. and E. coli isolates.
When 180 food samples were examined for Salmonella spp., 60.8% of the meat and 18.0% of the shellfish samples were positive, yielding 91 Salmonella isolates. E. coli was present in more than 90% of all food sources. Isolates were screened for antibiotic resistance against 15 antibiotics, and 50.5% of the Salmonella isolates were found to be resistant to at least one antibiotic, and 78 to 89% of these isolates from pork and poultry displayed this characteristic. On the other hand, 83.8% of E. coli isolates were resistant to at least one antibiotic, and the resistance rates for this group were 100% for pork, chicken, and chicken feces isolates, and the rates for beef isolates and shellfish isolates were 65% and 55%, respectively. In addition, multiresistance (resistance to at least three different classes of antibiotics) was detected in 20.9% of Salmonella isolates and in 61.6% of E. coli isolates. When 23 Salmonella isolates and 35 E. coli isolates were screened for class 1 integrons by PCR, 3/23 (13%) of the Salmonella isolates and 20/35 (57.1%) of the E. coli isolates were positive for the PCR amplification product of class 1 integrons, with six patterns (2,650, 2,000, 1,700, 1,500, 1,250, and 1,200 bp) detected. Restriction fragment length polymorphism analysis of the PCR products showed that isolates having the same amplicon sizes had the same restriction patterns, suggesting that the gene cassettes in these strains were likely to be identical (Fig. 1). Sequence analysis of the integron PCR products showed the presence of classic gene cassettes in the integrons, including aadA1, aadA2, and aadA5 (which confer resistance to streptomycin and spectinomycin), aacA4 (which confers resistance to aminoglycosides), the dihydrofolate reductase gene cassettes dhfrXII, dfrA1 and dhfrA17 (which confer resistance to trimethoprim), the beta-lactamase gene blaPSE1 (which confers resistance to ampicillin), and catB3 (which confers resistance to chloramphenicol). When isolates contained a gene cassette, the corresponding antibiotic resistance phenotypes were detected in most of the cases, except for the E/P/18a isolate, which contained the dhfrXII gene cassette with sensitivity to trimethoprim. Resistance to streptomycin in the presence of the aadA gene varied from full resistance to intermediate susceptibility (Table 1).
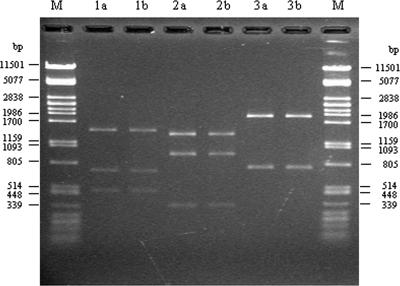
FIG. 1.
Restriction fragment length polymorphism analysis of 2.65-kb class 1 integron PCR product of isolates E/C/16a and E/C/21a. Lanes M, lambda DNA PstI marker; 1a, 2a, and 3a, digestion of integron PCR product of isolates E/C/16a with HincII, SspI, and SacII enzymes, respectively; 1b, 2b, and 3b, digestion of integron PCR product of isolate E/C/21a with HincII, SspI, and SacII enzymes, respectively.
TABLE 1.
Features of the E. coli and Salmonella isolates carrying class 1 integrons
| Integron size(s) (kb) | Isolate (species) | Source | Genes identified within integrons | Resistance phenotype pattern
|
|
|---|---|---|---|---|---|
| Full | Intermediate | ||||
| 2.0 | S/SF/8a (Salmonella serovar Typhimurium) | Shellfish | dhfrXII-orfF-aadA2 | TET, TMP, KAN | STR |
| 2.0 | S/P/9 (Salmonella serovar Typhimurium) | Pork | dhfrXII-orf-aadA2 | AMP, TET, GEN, SUL, TMP, STR, KAN, AMX | |
| 2.0 | E/C/24a (E. coli) | Chicken | N/Aa | AMP, TET, GEN, SUL, TMP, AMX, CHL, STR | CEF, AMC, NAL, ENR |
| 2.0 | E/SF/29 (E. coli) | Shellfish | N/A | AMP, TET, GEN, SUL, CEF, TMP, STR, NAL, AMX, CEF, AMC | CHL, ENR |
| 2.0 | E/F/2 (E. coli) | Chicken feces | N/A | AMP, TET, GEN, CHL, SUL, TMP, AMX | STR, KAN, NAL, ENR, AMC |
| 2.0 | E/F/8 (E. coli) | Chicken feces | N/A | AMP, TET, GEN, CHL, SUL, TMP, KAN, NAL, ENR, AMX | CEF, AMC, STR |
| 2.0 | E/F/25 (E. coli) | Chicken feces | N/A | TET, CHL, SUL, TMP, NOR, NAL, ENR, CIP | CEF, STR |
| 2.0 | E/P/18a (E. coli) | Pork | dhfrXII-orf-aadA2 | AMP, TET, GEN, SUL, NAL, ENR, AMX, STR | CEF, NOR |
| 2.0 | E/P/27a (E. coli) | Pork | N/A | CIP, TET, GEN, SUL, TMP, NOR, NAL, ENR, STR | CEF, AMC, AMP, AMX |
| 2.0 | E/P/43a (E. coli) | Pork | N/A | AMP, TET, GEN, SUL, TMP, AMX, STR | CHL |
| 1.5 | E/C/29a (E. coli) | Chicken | dfrA1-aadA1 | AMP, TET, SUL, TMP, AMX | CEF, AMC, STR |
| 1.5 | E/P/25a (E. coli) | Pork | N/A | AMP, CIP, TET, CHL, SUL, TMP, NOR, STR, NAL, ENR, AMX | CEF, AMC |
| 1.7 | E/SF/1a (E. coli) | Shellfish | dhfr17-aadA5 | AMP, TET, CHL, SUL, TMP, STR, NAL, AMX | CEF, AMC, ENR |
| 1.7 | E/C/15a (E. coli) | Chicken | N/A | AMP, TET, SUL, TMP, STR, NAL, ENR, AMX | CEF, AMC |
| 1.7 | E/C/17a (E. coli) | Chicken | dhfr17-aadA5 | AMP, TET, GEN, CHL, SUL, TMP, NOR, KAN, NAL, ENR, AMX, STR | CEF, CIP, AMC |
| 1.7 | E/F/9 (E. coli) | Chicken feces | N/A | AMP, TET, GEN, CHL, SUL, TMP, NAL, AMX, CEF | AMC, STR, ENR |
| 1.7 | E/F/13 (E. coli) | Chicken feces | N/A | TET, GEN, CHL, SUL, TMP, KAN, NAL | CEF, STR, ENR |
| 1.7 | E/F/16 (E. coli) | Chicken feces | N/A | TET, GEN, CHL, SUL, TMP, KAN, NAL | CEF, STR, ENR |
| 1.7 | E/F/20 (E. coli) | Chicken feces | N/A | TET, GEN, CHL, SUL, TMP, KAN, NAL | STR, ENR |
| 2.65 | E/C/16a (E. coli) | Chicken | aacA4-catB3-dfrA1 | AMP, CIP, TET, GEN, CHL, SUL, TMP, NOR, NAL, ENR, AMX, CEF, AMC | KAN |
| 2.65 | E/C/21a (E. coli) | Chicken | N/A | AMP, CIP, TET, GEN, CHL, SUL, TMP, NOR, STR, NAL, ENR, AMX, CEF | AMC, KAN |
| 1.2, 1.25 | S/C/21a (S. Albany) | Chicken | blaPSE1, dfrA1 | AMP, TET, CHL, SUL, TMP, NAL, AMX | |
| 2.0, 1.5 | E/F/3 (E. coli) | Chicken feces | dhfrXII-orf-aadA2, dfrA1-aadA1 | AMP, TET, GEN, CHL, SUL, TMP, STR, NAL, ENR, AMX | CEF, NOR |
N/A, not applicable.
Plasmid content of Salmonella and E. coli isolates.
The Kado and Liu method (28) was used to examine Salmonella and E. coli isolates for plasmids. The plasmid extraction of 23 antibiotic-resistant Salmonella isolates and 33 E. coli isolates showed that all of the isolates tested contained plasmids, and sizes ranged from less than 8 kb to more than 165 kb. Thirty-five percent of the Salmonella isolates and 76% of the E. coli isolates contained plasmids of more than 95 kb, and some of the isolates contained two large plasmids.
Transfer of antibiotic resistance genes in E. coli and Salmonella isolates by conjugation.
The antibiotic susceptibility test of transconjugants showed that the donors could transfer all or part of their resistance phenotypes to the recipients. The plasmid profiles of the donor, the recipient, and the transconjugants were studied for selective donor-recipient combinations. When isolate S/C/5a (Salmonella serovar Havana), which contained plasmids of 115 kb and 140 kb, was used as a donor, it was observed that transconjugants acquired either an AMP/AMX resistance phenotype or an AMP/AMX/SUL/GEN/CHL resistance phenotype, depending on whether the recipients obtained the 115-kb plasmid or the 140-kb plasmid from the donor. Similarly, when the E/C/4a and E/C/5a isolates, which both contained the 120-kb plasmid, were used as donors, the transconjugants were found to acquire the 120-kb plasmid and the corresponding antibiotic resistance phenotype (the AMP/AMX/SUL/GEN/CHL resistance phenotype or the AMP/AMX/SUL resistance phenotype, respectively) (Table 2 and Fig. 2). Therefore, these large plasmids were conjugative and contained many antibiotic resistance genes. It was also noticed in this study that in conjugation, the recipients could acquire plasmids from donors regardless of whether the recipients harbored their own plasmids or not; this observation shows furthermore that conjugation mechanisms could easily occur among the bacterial population.
TABLE 2.
Transfer of resistance phenotypes of selected conjugation tests
| Donors (species) | Resistance phenotype of donor | Resistance phenotype (plasmid size [kb])
|
|
|---|---|---|---|
| Transferred to E. coli JM109 E/P/8a, E/F/13, or E/F/16 | Transferred to E. coli HB101 | ||
| S/C/5 (Salmonella serovar Havana) | AMP, AMX, GEN, CHL, SUL | AMP, AMX (115) | N/A |
| S/C/5 (Salmonella serovar Havana) | AMP, AMX, GEN, CHL, SUL | AMP, AMX, GEN, CHL, SUL (140) | N/A |
| E/C/4a (E. coli) | AMP, AMX, CIP, TET, GEN, CHL, SUL, NOR, NAL, ENR | N/Ac | AMP, AMX, GEN, CHL, SUL (120)a |
| E/C/5a (E. coli) | AMP, AMX, CIP, TET, SUL, TMP, NOR, STR, NAL, ENR, AMC, CEF | N/A | AMP, AMX, SUL (120)b |
Transfer of the tetracycline phenotype could not be measured as the recipient was also resistant to this antibiotic before the conjugation experiment.
Transfer of the tetracycline and streptomycin resistance phenotypes could not be measured as the recipient was also resistant to these antibiotics before the conjugation experiment.
N/A, not applicable.
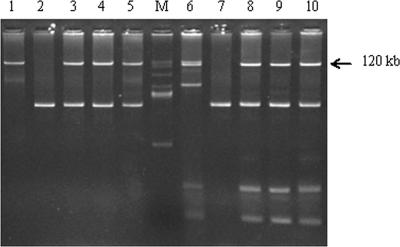
FIG. 2.
Transfer of plasmid DNA in conjugation experiments. Lanes M, BAC-Tracker supercoiled DNA ladder; 1, strain E/C/4a (donor); 2, E. coli HB101 (recipient); 3 to 5, transconjugation of E/C/4a with E. coli HB101; 6, strain E/C/5a (donor); 7, E. coli HB101 (recipient); 8 to 10, transconjugation of E/C/5a with E. coli HB101.
DISCUSSION
The results demonstrated that raw food samples were heavily contaminated with enteric bacteria. The rate of Salmonella contamination in retail meat samples above 60% is significantly higher than that reported for other countries (16, 19, 21, 25, 27, 45, 58, 61). The antibiotic resistance susceptibility results also indicated alarming multiresistance frequencies for Salmonella and E. coli isolates from food, where multiresistance rates of 20.9% for Salmonella isolates and 61.6% for E. coli isolates were detected and most probably reflect the unregulated use of antibiotics in food-producing animals in the country (60). It was found that 78 to 89% of the Salmonella spp. isolated from pork and poultry were resistant to one or more antibiotics. This level was higher than that obtained by Arvanitidou et al. (7) in Greece (58.1%) or by Seyfarth et al. (50) in Denmark (9.2 to 11.1%) but lower than that reported by Carraminana et al. (14) in Spain (100%). Class 1 integrons, the mobile elements that are known for the efficient spread of antibiotic resistance genes due to mobilization capabilities of gene cassettes (24, 46, 57), were detected in E. coli and Salmonella isolates. The finding that only 13% of Salmonella isolates contained class 1 integrons suggests that most of the resistant Salmonella isolates contained resistance elements other than integrons. In contrast, integron-mediated antibiotic resistance was common among E. coli isolates, where more than 50% of the isolates were shown to contain integrons, with some containing two integrons. Various gene cassettes detected in Salmonella and E. coli isolates in this study, including aadA1, aadA2, aadA5, aacA4, dhfrXII, dfrA1, dhfrA17, blaPSE1, and catB3, have been detected in clinical and nonclinical isolates in reports from many countries (6, 20, 29, 31, 32, 34, 35, 43, 49, 51, 52, 62, 64). The gene cassette array aacA4-catB3-dfrA1, which has been reported recently in Japan (31) and China (34), was also detected in E. coli isolates in this study, indicating that this resistance cassette is widespread in Asia.
The transfer of conjugative plasmids is considered to be the most common mechanism for genetic exchange between bacteria, as plasmid conjugation can occur at high frequency and is capable of the cotransfer of several resistance genes, and transfer can occur both within bacterial species and between different species (13, 47). This study demonstrated that plasmids were widely distributed in E. coli and Salmonella isolates collected from food in Vietnam and that these isolates contained large conjugative plasmids which contained many antibiotic resistance determinants. The presence of large conjugative resistance plasmids has been detected in Salmonella and E. coli isolates from food and food-producing animals in many countries (8, 10, 23, 38, 63). High-molecular-weight plasmids are often attributed to virulence and antibiotic resistance (48). Therefore, the presence of the large plasmids in E. coli and Salmonella isolates detected in this study could have contributed to the spread of resistance genes. Using different combinations of donor and recipient strains, it has also been demonstrated that resistance markers can be readily transferred among the same and different species (e.g., Salmonella spp. and E. coli). These findings demonstrate the importance of plasmids in the dissemination of antibiotic resistance genes in enteric bacteria in Vietnamese food samples.
In summary, results confirm the role of raw food as a reservoir of antibiotic resistance bacteria that contained a pool of mobile genetic elements, which are ready to disseminate antibiotic resistance genes to other human pathogens and so constitute a problem for human health. The application of hygiene practices along the food chain and the prudent use of antibiotics in animal husbandry are therefore essential. To control further emergence of antibiotic resistance, studies with comprehensive collections of samples are urgently needed to increase our understanding of molecular genetic mechanisms involved in the dissemination of antibiotic resistance genes from food-borne pathogens to humans.
Acknowledgments
We are grateful to Taghrid Istivan at RMIT University for her continued advice and help for the project.
T.T.H.V was supported by the Atlantic Philanthropies Foundation and the School of Applied Sciences, RMIT University.
Footnotes
Published ahead of print on 19 October 2007.
REFERENCES
- 1.Aarestrup, F. M. 1999. Association between the consumption of antimicrobial agents in animal husbandry and the occurrence of resistant bacteria among food animals. Int. J. Antimicrob. Agents 12:279-285. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ghamdi, M. S., F. El-Morsy, Z. H. Al-Mustafa, M. Al-Ramadhan, and M. Hanif. 1999. Antibiotic resistance of Escherichia coli isolated from poultry workers, patients and chicken in the eastern province of Saudi Arabia. Trop. Med. Int. Health 4:278-283. [DOI] [PubMed] [Google Scholar]
- 3.Angkititrakul, S., C. Chomvarin, T. Chaita, K. Kanistanon, and S. Waethewutajarn. 2005. Epidemiology of antimicrobial resistance in Salmonella isolated from pork, chicken meat and humans in Thailand. Southeast Asian J. Trop. Med. Public Health 36:1510-1515. [PubMed] [Google Scholar]
- 4.Angulo, F. J., K. R. Johnson, R. V. Tauxe, and M. L. Cohen. 2000. Origins and consequences of antimicrobial-resistant nontyphoidal Salmonella: implications for the use of fluoroquinolones in food animals. Microb. Drug Resist. 6:77-83. [DOI] [PubMed] [Google Scholar]
- 5.Anh, N. T., P. D. Cam, and A. Dalsgaard. 2001. Antimicrobial resistance of Shigella spp. isolated from diarrheal patients between 1989 and 1998 in Vietnam. Southeast Asian J. Trop. Med. Public Health 32:856-862. [PubMed] [Google Scholar]
- 6.Antunes, P., J. Machado, and L. Peixe. 2006. Characterization of antimicrobial resistance and class 1 and 2 integrons in Salmonella enterica isolates from different sources in Portugal. J. Antimicrob. Chemother. 58:297-304. [DOI] [PubMed] [Google Scholar]
- 7.Arvanitidou, M., A. Tsakris, D. Sofianou, and V. Katsouyannopoulos. 1998. Antimicrobial resistance and R-factor transfer of salmonellae isolated from chicken carcasses in Greek hospitals. Int. J. Food Microbiol. 14:197-201. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff, K. M., D. G. White, M. E. Hume, T. L. Poole, and D. J. Nisbet. 2005. The chloramphenicol resistance gene cmlA is disseminated on transferable plasmids that confer multiple-drug resistance in swine Escherichia coli. FEMS Microbiol. Lett. 243:285-291. [DOI] [PubMed] [Google Scholar]
- 9.Boonmar, S., A. Bangtrakulnonth, S. Pornruangwong, S. Samosornsuk, K. Kaneko, and M. Ogawa. 1998. Significant increase in antibiotic resistance of Salmonella isolates from human beings and chicken meat in Thailand. Vet. Microbiol. 62:73-80. [DOI] [PubMed] [Google Scholar]
- 10.Brackelsberg, C. A., L. K. Nolan, and J. Brown. 1997. Characterization of Salmonella dublin and Salmonella typhimurium (Copenhagen) isolates from cattle. Vet. Res. Commun. 21:409-420. [DOI] [PubMed] [Google Scholar]
- 11.Bywater, R. J. 2004. Veterinary use of antimicrobials and emergence of resistance in zoonotic and sentinel bacteria in the EU. J. Vet. Med. B 51:361-363. [DOI] [PubMed] [Google Scholar]
- 12.Cao, V., T. Lambert, D. Q. Nhu, H. K. Loan, N. K. Hoang, G. Arlet, and P. Courvalin. 2002. Distribution of extended-spectrum β-lactamases in clinical isolates of Enterobacteriaceae in Vietnam. Antimicrob. Agents Chemother. 46:3739-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carattoli, A. 2003. Plasmid-mediated antimicrobial resistance in Salmonella enterica. Curr. Issues Mol. Biol. 5:113-122. [PubMed] [Google Scholar]
- 14.Carraminana, J. J., C. Rota, I. Agustyn, and A. Herrera. 2004. High prevalence of multiple resistance to antibiotics in Salmonella serovars isolated from a poultry slaughterhouse in Spain. Vet. Microbiol. 104:133-139. [DOI] [PubMed] [Google Scholar]
- 15.Chiu, C.-H., T.-L. Wu, L.-H. Su, C. Chu, J.-H. Chia, A.-J. Kuo, M.-S. Chien, and T.-Y. Lin. 2002. The emergence in Taiwan of fluoroquinolone resistance in Salmonella enterica serotype Choleraesuis. N. Engl. J. Med. 346:413-419. [DOI] [PubMed] [Google Scholar]
- 16.Chung, Y. H., S. Y. Kim, and Y. H. Chang. 2003. Prevalence and antibiotic susceptibility of Salmonella isolated from foods in Korea from 1993 to 2001. J. Food Prot. 66:1154-1157. [DOI] [PubMed] [Google Scholar]
- 17.CLSI. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. CLSI/NCCLS M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 18.Davis, M. A., D. D. Hancock, T. E. Besser, D. H. Rice, J. M. Gay, C. Gay, L. Gearhart, and R. DiGiacomo. 1999. Changes in antimicrobial resistance among Salmonella enterica serovar Typhimurium isolates from humans and cattle in the Northwestern United States, 1982-1997. Emerg. Infect. Dis. 5:802-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez, C., I. Gomez, and J. Zumalacarregui. 2002. Prevalence of Salmonella and Campylobacter in retail chicken meat in Spain. Int. J. Food Microbiol. 72:165-168. [DOI] [PubMed] [Google Scholar]
- 20.Doublet, B., R. Lailler, D. Meunier, A. Brisabois, D. Boyd, M. Mulvey, R., E. Chaslus-Dancla, and A. Cloeckaert. 2003. Variant Salmonella genomic island 1 antibiotic resistance gene cluster in Salmonella enterica serovar Albany. Emerg. Infect. Dis. 9:585-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy, G., O. M. Cloak, M. G. O'Sullivan, A. Guillet, J. J. Sheridan, I. S. Blair, and D. A. McDowell. 1999. The incidence and antibiotic resistance profiles of Salmonella spp. on Irish retail meat products. Food Microbiol. 16:623-631. [Google Scholar]
- 22.Ehara, M., B. M. Nguyen, D. T. Nguyen, C. Toma, N. Higa, and M. Iwanaga. 2004. Drug susceptibility and its genetic basis in epidemic Vibrio cholerae O1 in Vietnam. Epidemiol. Infect. 132:595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebreyes, W. A., and S. Thakur. 2005. Multidrug-resistant Salmonella enterica serovar Muenchen from pigs and humans and potential interserovar transfer of antimicrobial resistance. Antimicrob. Agents Chemother. 49:503-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein, C., M. D. Lee, S. Sanchez, C. Hudson, P. Brad, B. Register, M. Grady, C. Liebert, A. O. Summers, D. G. White, and J. J. Maurer. 2001. Incidence of class 1 and 2 integrases in clinical and commensal bacteria from livestock, companion animals, and exotics. Antimicrob. Agents Chemother. 45:723-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison, W. A., C. J. Griffith, D. Tennant, and A. C. Peters. 2001. Incidence of Campylobacter and Salmonella isolated from retail chicken and associated packaging in South Wales. Lett. Appl. Microbiol. 33:450-454. [DOI] [PubMed] [Google Scholar]
- 26.Isenbarger, D. W., C. W. Hoge, A. Srijan, C. Pitarangsi, N. Vithayasai, L. Bodhidatta, K. W. Hickey, and P. D. Cam. 2002. Comparative antibiotic resistance of diarrheal pathogens from Vietnam and Thailand, 1996-1999. Emerg. Infect. Dis. 8:175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan, E., J. Egan, C. Dullea, J. Ward, K. McGillicuddy, G. Murray, A. Murphy, B. Bradshaw, N. Leonard, P. Rafter, and S. McDowell. 2006. Salmonella surveillance in raw and cooked meat and meat products in the Republic of Ireland from 2002 to 2004. Int. J. Food Microbiol. 112:66-70. [DOI] [PubMed] [Google Scholar]
- 28.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang, S.-G., D.-Y. Lee, S.-J. Shin, J.-M. Ahn, and H.-S. Yoo. 2005. Changes in patterns of antimicrobial susceptibility and class 1 integron carriage among Escherichia coli isolates. J. Vet. Sci. 6:201-205. [PubMed] [Google Scholar]
- 30.Keeling, S. E. 2002. Safety of wastewater for the irrigation of dairy farm. Ph.D. thesis. Royal Melbourne Institute of Technology (RMIT), Melbourne, Victoria, Australia.
- 31.Kumai, Y., Y. Suzuki, Y. Tanaka, K. Shima, R. K. Bhadra, S. Yamasaki, K. Kuroda, and G. Endo. 2005. Characterization of multidrug-resistance phenotypes and genotypes of Escherichia coli strains isolated from swine from an abattoir in Osaka, Japan. Epidemiol. Infect. 133:59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levings, R. S., D. Lightfoot, S. R. Partridge, R. M. Hall, and S. P. Djordjevic. 2005. The genomic island SGI1, containing the multiple antibiotic resistance region of Salmonella enterica serovar Typhimurium DT104 or variants of it, is widely distributed in other S. enterica serovars. J. Bacteriol. 187:4401-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy, S. B., G. B. FitzGerald, and A. B. Macone. 1976. Spread of antibiotic-resistant plasmids from chicken to chicken and from chicken to man. Nature 260:40-42. [DOI] [PubMed] [Google Scholar]
- 34.Li, X., L. Shi, W. Yang, L. Li, and S. Yamasaki. 2006. New array of aacA4-catB3-dfrA1 gene cassettes and a noncoding cassette from a class-1-integron-positive clinical strain of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:2278-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindstedt, B. A., E. Heir, I. Nygard, and G. Kapperud. 2003. Characterization of class I integrons in clinical strains of Salmonella enterica subsp. enterica serovars Typhimurium and Enteritidis from Norwegian hospitals. J. Med. Microbiol. 52:141-149. [DOI] [PubMed] [Google Scholar]
- 36.Majtan, V., L. Majtanova, and L. Kovac. 2004. Analysis of integrons in human isolates of Salmonella enterica serovar typhimurium isolated in the Slovak Republic. FEMS Microbiol. Lett. 239:25-31. [DOI] [PubMed] [Google Scholar]
- 37.Manie, T., S. Khan, V. S. Brözel, W. J. Veith, and P. A. Gouws. 1998. Antimicrobial resistance of bacteria isolated from slaughtered and retail chickens in South Africa. Lett. Appl. Microbiol. 26:253-258. [DOI] [PubMed] [Google Scholar]
- 38.Michael, G. B., M. Cardoso, and S. Schwarz. 2005. Class 1 integron-associated gene cassettes in Salmonella enterica subsp. enterica serovar Agona isolated from pig carcasses in Brazil. J. Antimicrob. Chemother. 55:776-779. [DOI] [PubMed] [Google Scholar]
- 39.NCCLS. 2004. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, 2nd ed. Approved standard M31-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 40.Nguyen, T. V., P. V. Le, C. H. Le, and A. Weintraub. 2005. Antibiotic resistance in diarrheagenic Escherichia coli and Shigella strains isolated from children in Hanoi, Vietnam. Antimicrob. Agents Chemother. 49:816-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NMKL. 1991. Salmonella bacteria. Detection in foods. Method no. 71, 4th ed. Nordic Committee on Food Analysis, Oslo, Norway.
- 42.NMKL. 1996. Thermotolerant coliform bacteria. Enumeration in foods. Method no. 125, 3rd ed. Nordic Committee on Food Analysis, Oslo, Norway.
- 43.Nogrady, N., I. Gado, A. Toth, and J. Paszti. 2005. Antibiotic resistance and class 1 integron patterns of non-typhoidal human Salmonella serotypes isolated in Hungary in 2002 and 2003. Int. J. Antimicrob. Agents 26:126-132. [DOI] [PubMed] [Google Scholar]
- 44.O'Brien, T. F., J. D. Hopkins, E. S. Gilleece, A. A. Medeiros, R. L. Kent, B. O. Blackburn, M. B. Holmes, J. P. Reardon, J. M. Vergeront, W. L. Schell, E. Christenson, M. L. Bissett, and E. V. Morse. 1982. Molecular epidemiology of antibiotic resistance in salmonella from animals and human beings in the United States. N. Engl. J. Med. 307:1-6. [DOI] [PubMed] [Google Scholar]
- 45.Plummer, R. A., S. J. Blissett, and C. E. Dodd. 1995. Salmonella contamination of retail chicken products sold in the UK. J. Food Prot. 58:843-846. [DOI] [PubMed] [Google Scholar]
- 46.Recchia, G. D., and R. M. Hall. 1995. Gene cassettes: a new class of mobile element. Microbiology 141:3015-3027. [DOI] [PubMed] [Google Scholar]
- 47.Rice, L. B., D. Sahm, and R. A. Bonomo. 2003. Mechanisms of resistance to antibacterial agents, p. 1074-1101. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 48.Rychlik, I., D. Gregorova, and H. Hradecka. 2006. Distribution and function of plasmids in Salmonella enterica. Vet. Microbiol. 112:1-10. [DOI] [PubMed] [Google Scholar]
- 49.Sáenz, Y., L. Brinas, E. Dominguez, J. Ruiz, M. Zarazaga, J. Vila, and C. Torres. 2004. Mechanisms of resistance in multiple-antibiotic-resistant Escherichia coli strains of human, animal, and food origins. Antimicrob. Agents Chemother. 48:3996-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seyfarth, A. M., H. C. Wegener, and N. Frimodt-Moller. 1997. Antimicrobial resistance in Salmonella enterica subsp. enterica serovar typhimurium from humans and production animals. J. Antimicrob. Chemother. 40:67-75. [DOI] [PubMed] [Google Scholar]
- 51.Su, J., L. Shi, L. Yang, Z. Xiao, X. Li, and S. Yamasaki. 2006. Analysis of integrons in clinical isolates of Escherichia coli in China during the last six years. FEMS Microbiol. Lett. 254:75-80. [DOI] [PubMed] [Google Scholar]
- 52.Sunde, M. 2005. Prevalence and characterization of class 1 and class 2 integrons in Escherichia coli isolated from meat and meat products of Norwegian origin. J. Antimicrob. Chemother. 56:1019-1024. [DOI] [PubMed] [Google Scholar]
- 53.Sunde, M., and M. Nordstrom. 2006. The prevalence of, associations between and conjugal transfer of antibiotic resistance genes in Escherichia coli isolated from Norwegian meat and meat products. J. Antimicrob. Chemother. 58:741-747. [DOI] [PubMed] [Google Scholar]
- 54.Taremi, M., S. D. M. Mehdi, L. Gachkar, S. MoezArdalan, K. Zolfagharian, and M. Reza Zali. 2006. Prevalence and antimicrobial resistance of Campylobacter isolated from retail raw chicken and beef meat, Tehran, Iran. Int. J. Food Microbiol. 108:401-403. [DOI] [PubMed] [Google Scholar]
- 55.Teuber, M. 2001. Veterinary use and antibiotic resistance. Curr. Opin. Microbiol. 4:493-499. [DOI] [PubMed] [Google Scholar]
- 56.Threlfall, E. J., L. R. Ward, J. A. Frost, and G. A. Willshaw. 2000. The emergence and spread of antibiotic resistance in food-borne bacteria. Int. J. Food Microbiol. 62:1-5. [DOI] [PubMed] [Google Scholar]
- 57.Tosini, F., P. Visca, I. Luzzi, A. M. Dionisi, C. Pezzella, A. Petrucca, and A. Carattoli. 1998. Class 1 integron-borne multiple-antibiotic resistance carried by IncFI and IncL/M plasmids in Salmonella enterica serotype Typhimurium. Antimicrob. Agents Chemother. 42:3053-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uyttendaele, M., P. De Troy, and J. Debevere. 1999. Incidence of Salmonella, Campylobacter jejuni, Campylobacter coli, and Listeria monocytogenes in poultry carcasses and different types of poultry products for sale on the Belgian retail market. J. Food Prot. 62:735-740. [DOI] [PubMed] [Google Scholar]
- 59.Van den Bogaard, A. E., and E. E. Stobberingh. 2000. Epidemiology of resistance to antibiotics. Links between animals and humans. Int. J. Antimicrob. Agents 14:327-335. [DOI] [PubMed] [Google Scholar]
- 60.Van Nhiem, D., P. Paulsen, W. Suriyasathaporn, F. J. Smulders, M. N. Kyule, M. P. Baumann, K. H. Zessin, and P. Hong Ngan. 2006. Preliminary analysis of tetracycline residues in marketed pork in Hanoi, Vietnam. Ann. N. Y. Acad. Sci. 1081:534-542. [DOI] [PubMed] [Google Scholar]
- 61.Van Pelt, W., H. van der Zee, W. J. B. Wannet, A. W. van de Giessen, D. J. Mevius, N. M. Bolder, R. E. Komijn, and Y. T. van Duynhoven. 2003. Explosive increase of Salmonella Java in poultry in the Netherlands: consequences for public health. Euro Surveill. 8:31-35. [DOI] [PubMed] [Google Scholar]
- 62.Vo, A. T. T., E. van Duijkeren, A. C. Fluit, W. J. B. Wannet, A. J. Verbruggen, H. M. E. Maas, and W. Gaastra. 2006. Antibiotic resistance, integrons and Salmonella genomic island 1 among nontyphoidal Salmonella serovars in The Netherlands. Int. J. Antimicrob. Agents 28:172-179. [DOI] [PubMed] [Google Scholar]
- 63.Yates, C. M., M. C. Pearce, M. E. J. Woolhouse, and S. G. B. Amyes. 2004. High frequency transfer and horizontal spread of apramycin resistance in calf faecal Escherichia coli. J. Antimicrob. Chemother. 54:534-537. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, H., L. Shi, L. Li, S. Guo, X. Zhang, S. Yamasaki, S.-i. Miyoshi, and S. Shinoda. 2004. Identification and characterization of class 1 integron resistance gene cassettes among Salmonella strains isolated from healthy humans in China. Microbiol. Immunol. 48:639-645. [DOI] [PubMed] [Google Scholar]