Abstract
The genus Brucella includes a number of species that are major animal pathogens worldwide and significant causes of zoonotic infections of humans. Traditional methods of identifying Brucella to the species level can be time-consuming, can be subjective, and can pose a hazard to laboratory personnel in the absence of suitable biocontainment facilities. Using a robust phylogenetic framework, a number of single-nucleotide polymorphisms (SNPs) that define particular species within the genus were identified. These SNPs were used to develop a multiplex SNP detection assay, based on primer extension technology, that can rapidly and unambiguously identify an isolate as a member of one of the six classical Brucella species or as a member of the recently identified marine mammal group.
Brucellosis is a major cause of disease in livestock worldwide, with substantial implications for animal welfare and economic output. Furthermore, as the most common zoonotic disease, it remains a significant public health concern (22). Although a few parts of the developed world have eradicated the disease by a combination of strict veterinary hygiene measures, monitoring programs, and improved food safety measures, it remains endemic in large areas. Human disease usually reflects occupational exposure or the consumption of unpasteurized dairy products and can lead to a chronic debilitating infection.
The causative organisms of brucellosis are members of the genus Brucella. Traditionally, the group is divided into six species with distinct host specificities (1), although at the genetic level, members of the genus are highly conserved (14, 32). Reflecting this, DNA hybridization experiments led to the traditional view of Brucella taxonomy being challenged and the group being described as monospecific (28). Practical considerations meant that this classification system never found widespread support, and recently, moves were made to return to the classical species designations (20). Of the currently recognized species, B. abortus is predominantly associated with bovine brucellosis, B. melitensis is predominantly associated with small ruminant brucellosis, B. canis is predominantly associated with canine brucellosis, B. ovis is predominantly associated with ram epididymitis, and B. neotomae has been reported only in the desert wood rat. The remaining species, B. suis, has a broader host range, being predominantly associated with porcine brucellosis (biovars 1, 2, and 3) but also being found in hares (biovar 2), rangifers (biovar 4), and rodents (biovar 5). More recently, novel Brucella isolates were detected in a variety of marine mammal species (7, 10, 12, 17, 23). These isolates clearly form new Brucella groups, although they have yet to receive formal taxonomic recognition (13a, 20).
Classically, Brucella isolates are divided into species (and biovars in the case of B. abortus, B. melitensis, and B. suis) by a biotyping procedure that assesses a range of cultural, phenotypic, and antigenic properties (1). While biotyping has long been the mainstay of Brucella epidemiology, there are a number of acknowledged drawbacks. As the discriminating changes between some groups can be very subtle, particularly at the biovar level, the method is rather subjective and requires substantial expertise and experience. In addition, as the method is culture dependent, in terms of both initial isolation and ongoing discriminatory tests, it is a lengthy procedure. The need for specialized and standardized reagents and containment facilities that should ideally be used for handling Brucella means that only a few laboratories in the world undertake this procedure.
Given these issues, there is a clear need for molecular tests that can rapidly and categorically identify Brucella isolates to the species level and beyond. One developing area in microbial typing and forensics is the use of single-nucleotide polymorphisms (SNPs) (5). The use of SNPs in microbial characterization has many advantages, including their unambiguous nature and the multitude of platforms that have been developed to assay such markers (24). As an example, a minimal set of six SNPs useful for dividing Mycobacterium tuberculosis into six deeply branching phylogenetic groups for use in strain differentiation was recently described (11). Other applications of SNP-based typing in diagnostic microbiology described recently include the differentiation of Burkholderia spp. (25); genotyping of Staphylococcus aureus (16, 19), Listeria monocytogenes (8), and Escherichia coli (15); identification and typing of Bacillus anthracis (9, 18, 26); differentiation of vaccine and wild-type strains of varicella-zoster virus (6); determination of Mycobacterium tuberculosis drug resistance (29, 33); and identification of members of Campylobacter jejuni clonal complexes (2).
We have recently described the population structure of the Brucella group using a multilocus sequence analysis (MLSA) approach based on nine distinct genomic fragments (32). From this work, it is clear that sequencing of these nine loci offers an excellent way of placing Brucella isolates within one of the currently recognized species. However, sequencing of nine fragments is somewhat tedious, is not always practical, and is clearly not a rapid diagnostic procedure. Nevertheless, use of the phylogenetic framework described here allowed the identification of SNPs that define distinct Brucella species. Here, we outline the development of a multiplex assay based on these “species-defining” SNPs that offers a rapid, practical, and unambiguous alternative to current approaches to identifying Brucella isolates at the species level.
MATERIALS AND METHODS
Identification of SNPs.
The identification of SNPs and validation of their distribution in the Brucella population have been described previously (32). SNPs used in development of the speciation assay described here are located in glk, omp25, and trpE.
PCR of target genes.
PCR products representing glk, omp25, and trpE were amplified from genomic DNA or crude methanol lysates using the primers pairs listed in Table 1, as described previously (32). PCR products were purified by passage through QIAquick PCR purification columns (QIAGEN) and eluted in 50 μl of elution buffer.
TABLE 1.
Primers used in the SNP assay to identify Brucella isolates at the species level
| Primer | Sequence |
|---|---|
| Target amplification | |
| glk up | 5′-TATGGAAMAGATCGGCGG-3′ |
| glk down | 5′-GGGCCTTGTCCTCGAAGG-3′ |
| omp25 up | 5′-ATGCGCACTCTTAAGTCTC-3′ |
| omp25 down | 5′-GCCSAGGATGTTGTCCGT-3′ |
| trpE up | 5′-GCGCGCMTGGTATGGCG-3′ |
| trpE down | 5′-CKCSCCGCCATAGGCTTC-3′ |
| Extension | |
| glk196R (glk-1344)a | 5′-CGCTAAGAATTTGYTCGCCGG-3′ |
| glk427R (glk-1557) | 5′-TTTTTTTTTTGAAAGGATGCGCACCGGGATGC-3′ |
| glk255R (glk-1403) | 5′-TTTTTTTTTTTTTTTTTTTTAGGGTGGGCGTGATCTTGTCGGC-3′ |
| omp151F (omp25-3627) | 5′-TGGCTATACCGGTCTTTACCTTGGCTA-3′ |
| omp239F (omp25-3715) | 5′-TTTTTTTTTTTTTTTTTCTTTGCTGGCTGGAACTTCCAG-3′ |
| trp290R (trpE-2858) | 5′-TTTTTTTTTTTTTTTTTTTTTTTTTTTTGAAACCTTGGCGCCCGTCTGG-3′ |
Primer name with designation of equivalent site in original multilocus sequencing description (32) in parentheses.
SNP primer extension assay.
The SNP determination assay was developed using the CEQ SNP primer extension kit (part no. 390280; Beckman Coulter) and a Beckman Coulter CEQ8800 genetic analyzer. A SNP-primer extension premix was prepared by mixing 210 μl each of 10× buffer, ddUTP, ddGTP, ddCTP, and ddATP and 105 μl of polymerase. The optimized multiplex assay was carried out as follows. The protocol uses 0.4 μl of each of the three PCR products diluted 1:2 or 1:5 in water (glk, omp25, and trpE) and 1 μl of SNP interrogation primer at 0.5 to 2.0 μM, giving a final concentration of 0.5 to 2 pmol per reaction. The SNP interrogation primers selected for the final assay are shown in Table 1. Primer concentrations used varied depending on the efficiency of each primer and were selected following preliminary work. A master mix consisting of 2 μl SNP-primer extension premix, 1 μl of primer mix (0.5 μM trp290R, 0.9 μM omp151F, 1.2 μM glk196R, 1.0 μM glk427R, 1.0 μM omp239F, 2.0 μM glk255R) was prepared and made up to 8.8 μl with deionized water. This was added to the 1.2 μl of diluted PCR products (0.4 μl × 3) to give a final volume of 10 μl. The extension reaction was carried out using the following thermal cycling conditions: an initial denaturation step at 96°C for 1 min was followed by 28 cycles of 96°C for 20 s, 64°C for 10 s, and 72°C for 30 s. Following thermal cycling, the extended product was treated with shrimp alkaline phosphatase (SAP) to remove unincorporated dideoxynucleoside triphosphates. In a separate tube, 1.5 U SAP (in 1.5 μl) and 2 μl deionized water were mixed with 1.5 μl of the primer extension reaction mix. This mix was then incubated at 37°C for 60 min, followed by 80°C for 15 min in a thermocycler.
SNP detection on the CEQ8800 genetic analyzer.
In order to detect extended products on the CEQ8800 genetic analyzer, an aliquot of the cleaned reaction mixture was diluted 1:10 in deionized water. A 0.5-μl aliquot of this dilution was added to 39 μl of Beckman SLS (formamide) and 0.1 μl of CEQ DNA Size Standard Kit 80 in Beckman 96-well plates. The mixes were covered with a drop of mineral oil and immediately processed using the Beckman standard run method SNP-1 (denaturation at 90°C; injection time, 30 s; injection voltage, 2 kV; run temperature, 50°C; run current, 6.0 kV; run time, 16 min). SNP locus tags were defined on the basis of the midpoint of migration variability seen during optimization (see Table 3), and SNPs were called automatically using default SNP analysis parameters in the Beckman software (slope threshold, 10; relative peak height threshold, 10; dye mobility calibration, SNP version 1).
TABLE 3.
Properties of interrogation primers included in the final multiplex SNP assay
| Extension primer | Site interrogateda | Species identified | Baseb (unique/common) | Primer size (bp) | Tmc (°C) | SNP locus tag (bp) |
|---|---|---|---|---|---|---|
| glk196R | glk-1344 | B. neotomae | C/Td | 21 | 71.4 | 18.9 |
| omp151F | omp25-3627 | B. abortus | T/C | 27 | 70.1 | 26.0 |
| glk427R | glk-1557 | B. ovis | T/C | 32 | 77.1 | 30.85 |
| omp239F | omp25-3715 | B. canis | A/C | 39 | 69.0 | 38.0 |
| glk255R | glk-1403 | B. melitensis | C/G | 43 | 77.5 | 45.1 |
| trp290R | trpE-2858 | Marine mammal | T/C | 49 | 75.5 | 50.25 |
Designation of site interrogated in original multilocus sequencing description (32).
Takes into account whether the primer is in the forward or reverse orientation such that the actual base observed in fragment analysis is listed.
Tm of primer excluding the polynucleotide tail.
C shared with B. abortus ST6 isolates.
RESULTS AND DISCUSSION
Identification of SNPs included in the assay.
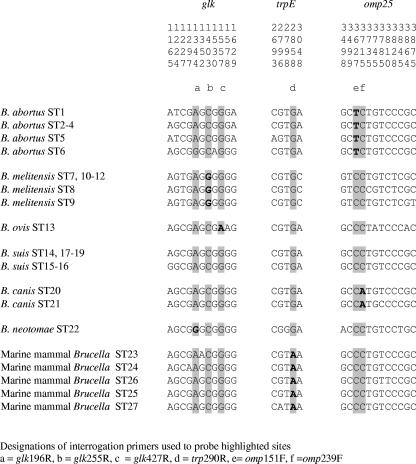
The rationale behind the assay described here was the selection of a series of species-specific SNPs that could be included in a multiplex assay facilitating the identification of an unknown Brucella isolate as a member of one of the six classically recognized Brucella species or the as-yet-unnamed marine mammal Brucella group. Species-specific SNPs were selected on the basis of sequencing of 4,396 bp corresponding to nine distinct genomic fragments from 160 strains representing all species and biovars (32). Although a number of SNPs could have been selected (see Table 4 in reference 32 for a full list), those that were found to perform best in initial optimization and included in the final assay were located in glk, trpE, and omp25. The SNPs selected are highlighted in Fig. 1 with reference to MLSA data described previously (32). The distribution and specificity of these SNPs in the Brucella population has since been further validated by sequencing of the equivalent fragments from a further 170 strains in addition to the 160 strains described previously (32) (data not shown).
FIG. 1.
Identification of SNPs that discriminate among Brucella species in glk, trpE, and omp25. The figure shows all sites within these fragments found to be polymorphic within 27 STs identified previously (32). The six sites chosen for interrogation in the multiplex SNP assay are highlighted, with the species-specific changes shown in boldface type. Residue numbering above the sequence relates to that reported previously (32), while the equivalent site designations used in this study, and as primer designations, are shown as a subscript.
The multiplex assay developed involves the interrogation of the base present at six SNP sites. Five of these have changes that, to date, are specific for a particular species or group. These markers are SNPs at position 151 of omp [omp151] for B. abortus (corresponding to omp25-3627 in reference 32), glk427 for B. ovis (glk-1557), omp239 for B. canis (omp25-3715), glk255 for B. melitensis (glk-1403), and trp290 for marine mammal Brucella (trpE-2858). The final marker, glk196 (corresponding to glk-1344 in reference 32), was originally thought to represent a change specific for B. neotomae, but this change was later found to be shared with members of B. abortus sequence type 6 (ST6). SNP markers specific for the B. suis group, as currently defined, were not identified by MLSA (32). As a result, members of this species are identified on the basis that they lack any of the species-specific markers and thus possess an overall multiplex profile that is distinct from any other currently described Brucella species. There are therefore eight predicted assay outcomes as shown in Table 2. Each species or group, with the exception of B. abortus, has a single predicted unique SNP profile. For B. abortus, there are two possible distinct profiles, labeled genotypes A and B, reflecting the sharing of an SNP between B. neotomae and B. abortus ST6 isolates. Genotype A corresponds to 107 of 110 B. abortus isolates examined to date by MLSA (our unpublished data). Genotype B is apparently much rarer in global terms, being characteristic of the B. abortus biovar 3 reference strain Tulya and a subset of African isolates belonging to ST6.
TABLE 2.
Specific SNP profiles differentiating members of the six Brucella species and the marine mammal Brucella isolates
| Organism | Profile for primera:
|
|||||
|---|---|---|---|---|---|---|
| glk196R (glk-1344) | omp151F (omp25-3627) | glk427R (glk-1557) | omp239F (omp25-3715) | glk255R (glk-1403) | trp290R (trpE-2858) | |
| B. abortus (genotype A) | T | T | C | C | G | C |
| B. abortus (genotype B) | C | T | C | C | G | C |
| B. melitensis | T | C | C | C | C | C |
| B. suis | T | C | C | C | G | C |
| B. ovis | T | C | T | C | G | C |
| B. canis | T | C | C | A | G | C |
| B. neotomae | C | C | C | C | G | C |
| Marine mammal Brucella | T | C | C | C | G | T |
Primer name with designation of equivalent site in original multilocus sequencing description (32) in parentheses.
Principle of the assay.
The assay described here was developed using the Beckman 8800 genetic analysis system but should be easily transferable to other fragment analysis platforms. The basis of the assay is a series of primer extension reactions. Following amplification of PCR products for the three genetic fragments of interest (omp25, glk, and trpE), there are three major steps to the assay. The first stage involves template clean-up by passage through a PCR clean-up column to remove deoxynucleoside triphosphates and primers. The second step involves single-base extension in which SNP interrogation primers anneal one base short of the target SNP and DNA polymerase inserts a dye terminator complementary to the SNP site. Finally, the extended product is treated with SAP to remove unincorporated dideoxynucleoside triphosphates before electrophoresis to identify and size the reaction products.
Assay optimization.
The final assay conditions presented in this study and described in Materials and Methods were established after the systematic variation of a large number of parameters including thermal cycling conditions (annealing temperature and length and number of cycles), the amounts of premix included in reaction mixtures, primer orientation, primer concentration, and dilution factor prior to electrophoresis. SNP interrogation primers were tested initially in individual reactions to assess performance, following which polynucleotide tails were added to selected primers to give suitable separation upon electrophoresis. Tailed interrogation primers were again tested individually to assess performance and validate migration size before the six primers were tested in multiplex reactions. The performance of the assay when carrying out the six SNP reactions individually and pooling products gave results comparable to those obtained true multiplexing, where all six primer extension reactions were carried out in a single tube, indicating that multiplexing was not compromising assay performance (data not shown).
The SNP interrogation primers included in the speciation assay range in size from 21 bp to 49 bp, although migration does not exactly correspond to primer size (Table 3). SNP interrogation primers were all separated by a migration gap of at least 4 bp to ensure ease of identification. As recommended by Beckman, the annealing temperature used for the generation of the primer extension product at 64°C was selected to be at least 5°C below the lowest primer midpoint temperature (Tm). It was necessary to include a degeneracy in interrogation primer gki196R, as during assay validation, the initial primer selected failed to generate an extension product, with members of ST23 found to have a polymorphism within the selected primer binding site (glk1352). The template DNA concentration recommended by Beckman (150 fmol per reaction) was used throughout initial optimization and validation. However, in order to avoid time-consuming quantification of PCR products prior to the primer extension assay, later work was carried out omitting this step. In the final routine assay procedure, 0.4 μl of purified PCR product diluted at 1:2 and 1:5 in water was used as a template. In our hands, both these dilutions consistently give good clean results, with peaks of suitable magnitude.
Assay performance and validation.
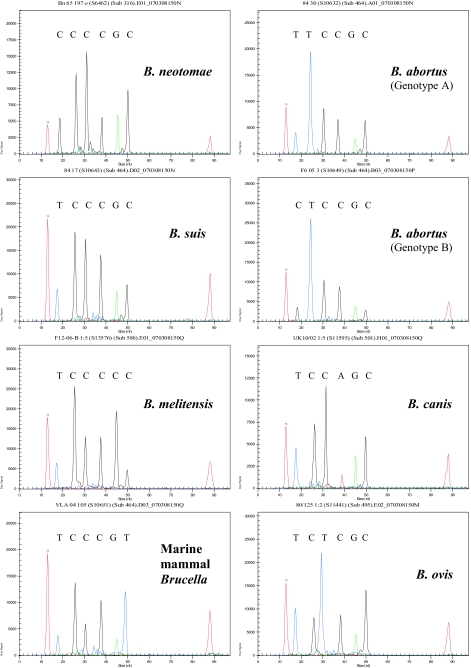
Figure 2 shows typical reaction profiles obtained with the multiplex assay demonstrating each of the eight specific profiles that the assay generates. To fully validate the assay, we examined the profiles of over 500 isolates including all species and biovar reference strains and a large number of field isolates. In all, 213 B. abortus (including 4 B. abortus isolates with the atypical genotype B profile), 111 B. melitensis, 42 B. suis, 8 B. canis, 39 B. ovis, 3 B. neotomae, and 94 marine mammal Brucella isolates were examined and found to give the predicted SNP profile. These isolates were part of a reference collection held at our laboratory that includes both historical isolates obtained from across the world over some 50 years as well as contemporary isolates coming through the laboratory as part of reference and surveillance activities. They therefore provide a robust test of assay scope. A small number of additional isolates (∼10) gave profiles that did not match the original species designation recorded in the strain archive or which appeared to represent a mixed-species signal. In all cases, examination of these isolates by full MLSA (32) and variable-number tandem-repeat typing (31) confirmed that either the original species designation was incorrect or the culture represented a mixed population.
FIG. 2.
Examples of the eight distinct genotypes identified by the SNP assay to identify isolates to the species level. The first and last peaks represent size standards of 13 and 88 bp, respectively. The six peaks in each assay relate to glk196, omp151, glk427, omp239, glk255, and trp290, moving from the smallest to the largest fragment. The colors of the peaks reflect different bases, where T is blue, C is black, G is green, and A is red. nt, nucleotide.
Specificity.
The currently accepted closest relatives of the Brucella group are members of the genus Ochrobactrum (27). In order to assess the specificity of the assay for Brucella, it was applied to type strains of five Ochrobactrum species (O. tritici LMG18957T, O. intermedium LMG3301T, O. gallinifaecis DSM15295T, O. anthropi LMG3331T, and O. grignonense LMG18954T). All three of the PCR products required for the SNP assay to identify isolates at the species level could be amplified from only two of these five strains (O. grignonense and O. intermedium) under the conditions examined in this study. Furthermore, when tested in the multiplex SNP interrogation assay, neither of these strains gave a complete profile. Sequencing of the PCR products allowed an examination of the regions corresponding to the interrogation primers used in the assay to identify isolates to the species level. This confirmed that there are an average of four mismatches between SNP interrogation primer sequences and the corresponding sequence in these two Ochrobactrum strains and likely explains the inability to generate a profile from these isolates. Thus, the assay appears to be specific for the Brucella group as currently defined, giving no result with the closest phylogenetic neighbors.
Conclusions.
The assay described here represents a novel approach to identifying Brucella isolates to species level. SNPs are particularly attractive assay targets, as Brucella isolates represent such a genetically conserved group (13, 14, 28, 32). Reflecting this, SNPs are likely to have occurred only once in evolution and are unlikely to mutate to new states or back to their ancestral state. The assay has a number of potential advantages. It overcomes the subjectivity associated with biotyping with an approach that is technically straightforward and has substantial advantages in terms of speed. It is easily possible to complete the entire assay in a day, as opposed to the several days currently required for biotyping following isolation of a pure culture. In addition, techniques that reduce potential exposure to live organisms are desirable given that brucellosis is easily acquired by laboratory personnel in the absence of stringent biocontainment facilities. We have also shown that the technique is applicable to crude extracts of material, overcoming the need for DNA preparation as required by molecular typing methods such as amplified fragment length polymorphism analysis (30) or insertion sequence-based fingerprinting (21). Indeed, as a PCR-based method, the technique should theoretically be directly applicable to field material, obviating the need for any culture prior to typing. The principle of the assay could also be readily transferred to any of the many alternative techniques applicable to SNP determination (24). In contrast to some existing and well-established molecular methods such as AMOS PCR (3, 4) that fail to detect all species and biovars, SNP speciation is all-encompassing, identifying all biovars of all known Brucella species.
The technique also lends itself to further expansion. There is clear potential for the current scheme to identify isolates to the species level to be extended in the future to identify vaccine strains and to subtype further to the level of biovar. Furthermore, should new species of Brucella be identified, it should be possible to determine suitable species-specific SNPs based on the existing MLSA scheme and incorporate additional SNP markers into the assay, allowing the identification of these groups.
Acknowledgments
All work described in this study was funded by the VLA Seedcorn Programme (grant SC0139).
We gratefully acknowledge the assistance of the VLA Sequencing Unit, and particularly Paula Keyes, in this project; Jo Craggs of Beckman Coulter for technical advice provided in the course of this study; and James Edwards-Smallbone for assisting with Ochrobactrum work.
Footnotes
Published ahead of print on 21 September 2007.
This paper is dedicated to the memory of Julie C. Scott.
REFERENCES
- 1.Alton, G. G., L. M. Jones, R. D. Angus, and J. M. Verger. 1988. Techniques for the brucellosis laboratory. INRA, Paris, France.
- 2.Best, E. L., A. J. Fox, J. A. Frost, and F. J. Bolton. 2005. Real-time single-nucleotide polymorphism profiling using Taqman technology for rapid recognition of Campylobacter jejuni clonal complexes. J. Med. Microbiol. 54:919-925. [DOI] [PubMed] [Google Scholar]
- 3.Bricker, B. J., and S. M. Halling. 1994. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J. Clin. Microbiol. 32:2660-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bricker, B. J., and S. M. Halling. 1995. Enhancement of the Brucella AMOS PCR assay for differentiation of Brucella abortus vaccine strains S19 and RB51. J. Clin. Microbiol. 33:1640-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budowle, B., M. D. Johnson, C. M. Fraser, T. J. Leighton, R. S. Murch, and R. Chakraborty. 2005. Genetic analysis and attribution of microbial forensics evidence. Crit. Rev. Microbiol. 31:233-254. [DOI] [PubMed] [Google Scholar]
- 6.Campsall, P. A., N. H. Au, J. S. Prendiville, D. P. Speert, R. Tan, and E. E. Thomas. 2004. Detection and genotyping of varicella-zoster virus by TaqMan allelic discrimination real-time PCR. J. Clin. Microbiol. 42:1409-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavareau, C., V. Wellemans, K. Walravens, M. Tryland, J. M. Verger, M. Grayon, A. Cloeckaert, J. J. Letesson, and J. Godfroid. 1998. Phenotypic and molecular characterization of a Brucella strain isolated from a minke whale (Balaenoptera acutorostrata). Microbiology 144:3267-3273. [DOI] [PubMed] [Google Scholar]
- 8.Ducey, T. F., B. Page, T. Usgaard, M. K. Borucki, K. Pupedis, and T. J. Ward. 2007. A single-nucleotide polymorphism-based multilocus genotyping assay for subtyping lineage I isolates of Listeria monocytogenes. Appl. Environ. Microbiol. 73:133-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Easterday, W. R., M. N. Van Ert, T. S. Simonson, D. M. Wagner, L. J. Kenefic, C. J. Allender, and P. Keim. 2005. Use of single nucleotide polymorphisms in the plcR gene for specific identification of Bacillus anthracis. J. Clin. Microbiol. 43:1995-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewalt, D. R., J. B. Payeur, B. M. Martin, D. R. Cummins, and W. G. Miller. 1994. Characteristics of a Brucella species from a bottlenose dolphin (Tursiops truncatus). J. Vet. Diagn. Investig. 6:448-452. [DOI] [PubMed] [Google Scholar]
- 11.Filliol, I., A. S. Motiwala, M. Cavatore, W. Qi, M. H. Hazbon, M. Bobadilla del Valle, J. Fyfe, L. Garcia-Garcia, N. Rastogi, C. Sola, T. Zozio, M. I. Guerrero, C. I. Leon, J. Crabtree, S. Angiuoli, K. D. Eisenach, R. Durmaz, M. L. Joloba, A. Rendon, J. Sifuentes-Osornio, A. Ponce de Leon, M. D. Cave, R. Fleischmann, T. S. Whittam, and D. Alland. 2006. Global phylogeny of Mycobacterium tuberculosis based on single nucleotide polymorphism (SNP) analysis: insights into tuberculosis evolution, phylogenetic accuracy of other DNA fingerprinting systems, and recommendations for a minimal standard SNP set. J. Bacteriol. 188:759-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster, G., K. L. Jahans, R. J. Reid, and H. M. Ross. 1996. Isolation of Brucella species from cetaceans, seals and an otter. Vet. Rec. 138:583-586. [DOI] [PubMed] [Google Scholar]
- 13.Gee, J. E., B. K. De, P. N. Levett, A. M. Whitney, R. T. Novak, and T. Popovic. 2004. Use of 16S rRNA gene sequencing for rapid confirmatory identification of Brucella isolates. J. Clin. Microbiol. 42:3649-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Groussaud, P., S. J. Shankster, M. S. Koylass, and A. M. Whatmore. 2007. Molecular typing divides marine mammal strains of Brucella into at least three groups with distinct host preferences. J. Med. Microbiol. 56:1512-1518. [DOI] [PubMed] [Google Scholar]
- 14.Halling, S. M., B. D. Peterson-Burch, B. J. Bricker, R. L. Zuerner, Z. Qing, L. L. Li, V. Kapur, D. P. Alt, and S. C. Olsen. 2005. Completion of the genome sequence of Brucella abortus and comparison to the highly similar genomes of Brucella melitensis and Brucella suis. J. Bacteriol. 187:2715-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hommais, F., S. Pereira, C. Acquaviva, P. Escobar-Paramo, and E. Denamur. 2005. Single-nucleotide polymorphism phylotyping of Escherichia coli. Appl. Environ. Microbiol. 71:4784-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huygens, F., J. Inman-Bamber, G. R. Nimmo, W. Munckhof, J. Schooneveldt, B. Harrison, J. A. McMahon, and P. M. Giffard. 2006. Staphylococcus aureus genotyping using novel real-time PCR formats. J. Clin. Microbiol. 44:3712-3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahans, K. L., G. Foster, and E. S. Broughton. 1997. The characterisation of Brucella strains isolated from marine mammals. Vet. Microbiol. 57:373-382. [DOI] [PubMed] [Google Scholar]
- 18.Keim, P., M. N. Van Ert, T. Pearson, A. J. Vogler, L. Y. Huynh, and D. M. Wagner. 2004. Anthrax molecular epidemiology and forensics: using the appropriate marker for different evolutionary scales. Infect. Genet. Evol. 4:205-213. [DOI] [PubMed] [Google Scholar]
- 19.McDonald, M., A. Dougall, D. Holt, F. Huygens, F. Oppedisano, P. M. Giffard, J. Inman-Bamber, A. J. Stephens, R. Towers, J. R. Carapetis, and B. J. Currie. 2006. Use of a single-nucleotide polymorphism genotyping system to demonstrate the unique epidemiology of methicillin-resistant Staphylococcus aureus in remote aboriginal communities. J. Clin. Microbiol. 44:3720-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osterman, B., and I. Moriyón. 2006. International Committee on Systematics of Prokaryotes. Subcommittee on the Taxonomy of Brucella. Report of the meeting, 17 September 2003, Pamplona, Spain. Int. J. Syst. Evol. Microbiol. 56:1173-1175. [Google Scholar]
- 21.Ouahrani, S., S. Michaux, J. Sri Widada, G. Bourg, R. Tournebize, M. Ramuz, and J. P. Liautard. 1993. Identification and sequence analysis of IS6501, an insertion sequence in Brucella spp.: relationship between genomic structure and the number of IS6501 copies. J. Gen. Microbiol. 139:3265-3273. [DOI] [PubMed] [Google Scholar]
- 22.Pappas, G., P. Papadimitriou, N. Akritidis, L. Christou, and E. V. Tsianos. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91-99. [DOI] [PubMed] [Google Scholar]
- 23.Ross, H. M., G. Foster, R. J. Reid, K. L. Jahans, and A. P. MacMillan. 1994. Brucella species infection in sea-mammals. Vet. Rec. 134:359. [DOI] [PubMed] [Google Scholar]
- 24.Sobrino, B., M. Brion, and A. Carracedo. 2005. SNPs in forensic genetics: a review on SNP typing methodologies. Forensic Sci. Int. 154:181-194. [DOI] [PubMed] [Google Scholar]
- 25.U'Ren, J. M., M. N. Van Ert, J. M. Schupp, W. R. Easterday, T. S. Simonson, R. T. Okinaka, T. Pearson, and P. Keim. 2005. Use of a real-time PCR TaqMan assay for rapid identification and differentiation of Burkholderia pseudomallei and Burkholderia mallei. J. Clin. Microbiol. 43:5771-5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Ert, M. N., W. R. Easterday, T. S. Simonson, J. M. U'Ren, T. Pearson, L. J. Kenefic, J. D. Busch, L. Y. Huynh, M. Dukerich, C. B. Trim, J. Beaudry, A. Welty-Bernard, T. Read, C. M. Fraser, J. Ravel, and P. Keim. 2007. Strain-specific single-nucleotide polymorphism assays for the Bacillus anthracis Ames strain. J. Clin. Microbiol. 45:47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velasco, J., C. Romero, I. Lopez-Goni, J. Leiva, R. Diaz, and I. Moriyon. 1998. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int. J. Syst. Bacteriol. 48:759-768. [DOI] [PubMed] [Google Scholar]
- 28.Verger, J. M., F. Grimont, P. A. D. Grimont, and M. Grayon. 1985. Brucella, a monospecific genus as shown by deoxyribonucleic acid hybridization. Int. J. Syst. Bacteriol. 35:292-295. [Google Scholar]
- 29.Wada, T., S. Maeda, A. Tamaru, S. Imai, A. Hase, and K. Kobayashi. 2004. Dual-probe assay for rapid detection of drug-resistant Mycobacterium tuberculosis by real-time PCR. J. Clin. Microbiol. 42:5277-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whatmore, A. M., T. J. Murphy, S. Shankster, E. Young, S. J. Cutler, and A. P. MacMillan. 2005. Use of amplified fragment length polymorphism to identify and type Brucella isolates of medical and veterinary interest. J. Clin. Microbiol. 43:761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whatmore, A. M., S. Shankster, L. L. Perrett, T. J. Murphy, S. D. Brew, R. E. Thirlwall, S. J. Cutler, and A. P. MacMillan. 2006. Identification and characterization of variable number of tandem repeat markers for typing of Brucella spp. J. Clin. Microbiol. 44:1982-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whatmore, A. M., L. L. Perrett, and A. P. MacMillan. 2007. Characterisation of the genetic diversity of Brucella by multilocus sequencing. BMC Microbiol. 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yesilkaya, H., F. Meacci, S. Niemann, D. Hillemann, S. Rusch-Gerdes, M. R. Barer, P. W. Andrew, and M. R. Oggioni. 2006. Evaluation of molecular-beacon, TaqMan, and fluorescence resonance energy transfer probes for detection of antibiotic resistance-conferring single nucleotide polymorphisms in mixed Mycobacterium tuberculosis DNA extracts. J. Clin. Microbiol. 44:3826-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]