Why do only certain microbes have the capacity to be virulent, and why are certain microbes virulent only in certain hosts? These fundamental questions have shaped and directed the thinking of microbiologists and the field of microbial pathogenesis since the germ theory of disease was proposed in the late 19th century. The germ theory gave rise to the prevailing view that there are fundamental differences between pathogenic and nonpathogenic microbes. This view, which regards virulence as a microbial characteristic, is supported by the observation that certain microbes require specific factors, such as toxins and capsules, for animal virulence. However, almost since it was first put forth that pathogens and nonpathogens are fundamentally different, there have been exceptions and challenges to this view. The demonstration that animal and/or in vitro passage can alter virulence illustrated that virulence is not necessarily a stable or invariant trait. Then, the 20th-century emergence of commensal microbes that were previously considered to be avirulent, such as Candida albicans (7) and Staphylococcus epidermidis (40), as clinically relevant pathogens provided clear evidence that virulence can be a function of the immune status of the host. For microbes that cause disease at one time but not another, the determinant of disease is often a change in the host that alters the host-microbe interaction. Hence, while virulence is a microbial property, it is expressed only in a susceptible host (4).
Given that the outcome of microbial infection is often a function of the immune status of the host and that a susceptible host is required for microbial virulence, it makes sense to categorize microbial pathogens based on how they are acquired. There are two basic types of acquisition. One type encompasses microbes that are acquired from other living hosts, including those of the same or different species, e.g., insects or protozoa. The other type encompasses microbes that are acquired from the environment, though it may contain the remains of formerly living species, such as decaying vegetation.
Microbes acquired from other hosts are adapted for host survival. Their virulence often stems from an altered host-microbe relationship in which the immune response cannot control microbial proliferation. Among human eukaryotic pathogens, Candida albicans, Pneumocystis spp., and the dermatophytes are each host acquired. The diseases caused by these microbes usually occur when an alteration in the host-microbe relationship, such as that induced by antibiotics, immunosuppression, or changes in their niche, results in a larger fungal burden. However, a large inoculum could also have the capacity to cause disease in healthy hosts, as illustrated by the dramatic example of self-experimentation in which a physician imbibed a C. albicans suspension that resulted in candidemia and candiduria (22). A similar example of self-experimentation led to the association of Helicobacter pylori, another human commensal microbe, with peptic ulcer disease.
In contrast, microbes acquired from the environment have no obvious requirement for residence in an animal host to survive or replicate. For example, and in contrast to the requirements of many parasites, there is no evidence that environmentally acquired pathogenic fungi, such as Cryptococcus neoformans and Histoplasma capsulatum, require residence in a living animal host. Although we acknowledge that some environmental fungi, such as H. capsulatum and Coccidioides spp., can be recovered from small animals, such as wild bats (37) and small rodents (9), respectively, their presence in such hosts does not appear to be a necessity for survival. Microbes acquired from the environment often cause disease in situations in which the host is exposed to a large microbial inoculum. For example, histoplasmosis and coccidioidomycosis in healthy hosts can follow cave visits (23) and archeological excavations (31), respectively. Nonetheless, the diseases caused by most environmentally acquired fungi occur predominantly in hosts with preexisting immune impairment and/or those that acquire large inocula. For example, coccidioidomycosis can develop in healthy individuals after exposure to a large inoculum and is generally self limited. However, in hosts with impaired immunity, coccidioidomycosis is a chronic, often lethal disease. In general, microbes acquired directly from the environment are not communicable to other hosts. Nevertheless, occasional person-to-person transmission of cryptococcosis has been reported as a consequence of needlestick accidents (18), showing that in unusual circumstances microbes acquired directly from the environment can be passed from host to host.
VIRULENCE OF HOST-ASSOCIATED FUNGI
The host-acquired fungi that are most often associated with human diseases are Candida spp., Pneumocystis spp., and the dermatophytes. Although each of these organisms can be found in healthy, asymptomatic hosts, disease is almost invariably associated with changes in host immunity or the host-microbe relationship. For example, human candidiasis occurs in the setting of reduced barrier or mucosal immunity, such as that which occurs following surgery, the placement of intravenous catheters, radiation-induced damage, and/or when immune responses are impaired, as in the setting of human immunodeficiency virus infection, corticosteroid therapy, and congenital defects. Similarly, pneumocystosis most often occurs in patients with impaired immunity, such as those with AIDS or medically induced immunosuppression. Many dermatophytes are part of the normal flora, yet they can be associated with disease in conditions in which the host is altered. For example, toe dermatophyte disease (e.g., athlete's foot) is associated with footwear that covers the skin surfaces and increases skin humidity and temperature, thereby promoting growth of the fungus, which in turn facilitates damage and disease. However, wearing shoes is not a sufficient condition for foot dermatophyte disease, a fact that implies that infection with certain strains, increased host susceptibility, or both could contribute to the amount of damage that can ensue in a given host.
The virulence of host-acquired pathogenic microbes is often associated with an immune response that can contribute to host damage. This is exemplified by the use of adjunctive corticosteroids in the treatment of Pneumocystis pneumonia, even though the disease occurs almost exclusively in the setting of an immune deficiency. Although this seems to be a paradox, adjunctive corticosteroids are given to reduce the damage that can result from the exuberant but ineffective inflammatory response that can be induced when the immune system fails to control fungal growth. In vaginal candidiasis, host damage can be associated with an altered host-microbe relationship that results in a neutrophil influx into the vagina (12).
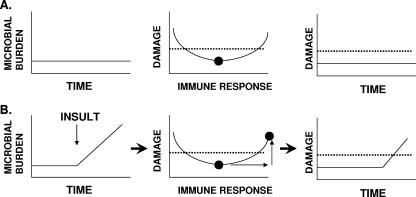
The association between antibiotic use and the development of candidiasis provides further evidence for a relationship between microbial burden and host damage. With candidiasis, antimicrobials alter the normal bacterial-fungal equilibrium by reducing the number of commensal bacteria, resulting in an excessive proliferation of yeast cells. Along the same lines, the absence or a low burden of Pneumocystis jirovecii organisms in healthy hosts is thought to be attributable to immune control (39). Hence, it is logical to posit that in patients with AIDS, the pathogenesis of Pneumocystis pneumonia involves an initial proliferation of P. jirovecii organisms, which then induces lung inflammation and damage stemming from the immune response to fungal beta-glucan (1). As such, the transition from the state of commensalism to that of disease results from host damage, which is triggered by an immune response to microbial antigens when the number of organisms exceeds that of the normal host-microbe relationship (Fig. 1).
FIG. 1.
Disease as a function of microbial burden as viewed from the damage response framework of microbial pathogenesis (5). (A) For host-microbe interactions resulting in the commensal state, there is a relatively constant microbial population over time that is maintained in its ecological niche by competition with other microbes and host defense (left panel). In the state of commensalism, the intensity of the immune response is such that it minimizes damage from the host-microbe interaction (middle panel), over time (right panel), and the damage incurred by the host is below the threshold for disease (dotted line). (B) When host-microbe interactions are perturbed, the microbial burden can increase (left panel), triggering a stronger immune response that can damage the host (middle panel) and eventually result in disease over time (right panel). The diagrams in panel B are what might be expected when a person takes antibacterial drugs and develops mucosal candidiasis. The drugs kill bacteria and perturb the ecological site, resulting in a proliferation of Candida spp. that elicits a stronger immune response, which in turn brings forth inflammation that causes sufficient damage to produce symptoms of disease.
VIRULENCE OF ENVIRONMENTALLY ACQUIRED PATHOGENS
In contrast to host-acquired pathogenic microbes, those that are acquired from the environment display virulence properties that were selected in a very different ecological niche. Before delving into this area, it is important to define the environment. By “environment,” we mean all those conditions, circumstances, and animate and inanimate objects which might be encountered by a host. Many environmental microbes are already associated with other microbes and microscopic hosts at the time they encounter humans. For example, some environmentally acquired pathogens, like Legionella pneumophila, may be acquired with an environmental host, such as an amoeba (41). Although there is no evidence that fungal pathogens are so acquired, some may be acquired shortly after interaction with another host, with the caveat that at the time of fungal acquisition, the former host is no longer living. The human-pathogenic fungi that are acquired from the environment occupy ecological niches defined by soils, trees, and decaying vegetation. Infection with C. neoformans, H. capsulatum, and Aspergillus spp. is believed to occur when hosts inhale fungal spores that are suspended in the air. Fungal spores are ideally suited for airborne dissemination and have dimensions appropriate for alveolar deposition (17). However, the apparent suitability of such spores for mammalian inhalation should not be construed as evidence for the design of these structures for animal infection. In fact, it is more likely that the ability of these spores to be carried by air currents reflects an adaptation for airborne dissemination that happens to also be suitable for pulmonary infection. Such a phenomenon provides support for the concept of “accidental virulence” (see below).
SOILS AS EXTREME ENVIRONMENTS
Soils are extreme environments, and soil-dwelling microbes must adapt to rapidly changing, harsh conditions. Soils contain a multitude of microbial species, estimated to be >4,000 species/gram soil (27). Soil microbes occupy an environment where there must be fierce competition for nutrients. Soils are constantly buffeted by changing climatic conditions, and soil-dwelling microbes must survive periods of inundation and desiccation and, in some climates, temperatures that range from freezing to scorching heat within a few hours. Depending on the density of vegetation and shading, microbes in the topsoil may also experience intense solar radiation. In addition to these nutritional and physical stresses, soil-dwelling microbes must cope with predators in the form of amoebae and other protista, which feed on bacteria and yeasts. Consequently, soil-dwelling microbes must develop ways to escape phagocytosis and/or survive ingestion through mechanisms for intracellular survival. In addition to predatory protozoa, there are predatory bacteria, phages, viruses, and small animals, such as nematodes, that pose threats to soil-dwelling microbes. Hence, soils are extreme environments in which survival almost certainly requires numerous adaptations to cope with life-threatening physical and biological processes. Some of these adaptations can also function in virulence (2).
VIRULENCE FACTOR PARADOX
One of the perplexing problems in microbial pathogenesis is that normally commensal microorganisms can be pathogenic under conditions in which the host-microbe relationship is disturbed, despite failing to meet criteria defined by Koch's original or molecular postulates (10, 11). It has been difficult to identify virulence factors that meet the classical criteria of traits needed for virulence but that do not also affect growth in vitro among commensal microbes, such as C. albicans (19). In contrast, many environmental microbes with no apparent need for an animal host in their life cycles express virulence factors that meet the criteria of the molecular postulates (33). For example, neither the capsule of C. neoformans nor the BAD1 adhesin of Blastomyces dermatitidis is needed for in vitro growth, yet both attributes are required for virulence in animal hosts (6, 21). One approach to understanding this paradox is to view these organisms in the context of their normal ecological niches. Given that C. albicans causes disease in the host that it normally inhabits, it does not need specialized attributes that permit host survival. In fact, for Candida spp., the most significant factor determining their capacity for virulence is the state of the host (42). In contrast, the virulence attributes of environmentally acquired pathogenic microbes, such as C. neoformans and B. dermatitidis, probably originated for environmental survival with their potential for animal pathogenicity having arisen from other causes (3).
“DUAL-USE” CONCEPT OF VIRULENCE FACTORS
The concept of “dual-use” virulence factors was originally introduced to explain the observation that some virulence factors of environmentally acquired microbes also promoted microbial survival in the environment (2, 3). For example, the capsule of Cryptococcus neoformans, which enhances animal virulence by inhibiting phagocytosis, also inhibits the phagocytosis of yeast cells by environmental amoebae and protects against desiccation (3). Similarly, melanin, which protects against environmental stresses, is also associated with enhanced animal virulence (3, 29). Notably, the concept of dual-use virulence factors might also extend to host-acquired microbes when/if microbial determinants that enhance virulence are those that are required for survival in the commensal state. In this regard, the host is in effect the “environment” for host-acquired microbes. However, Koch's postulates (10) and the later molecular postulates (11) may not be applicable to (commensal) organisms that require specific and noninterchangeable niches. For these organisms, growth, survival, and virulence might not be separable, whereas they may be discrete qualities for environmentally acquired microbes that can occupy multiple ecological niches. One apparent exception to this generalization is Aspergillus fumigatus, which is acquired from the environment and is pathogenic primarily because of host impairment (38). However, unlike other pathogenic, environmentally acquired fungi, A. fumigatus appears to lack classical virulence factors. This may reflect redundancy in proteolytic enzymes, antioxidant mechanisms, etc., which serve to ensure its survival in the environment. In this regard, Aspergillus fumigatus resembles other environmentally acquired fungi in having dual-use attributes that promote survival in the environment and animal virulence. However, it is noteworthy that in addition to causing aspergillosis in immunocompromised hosts, Aspergillus spp. also cause disease at the other end of the immunological spectrum when overly exuberant immune responses produce allergic bronchopulmonary aspergillosis.
“CARDS” OF VIRULENCE
When considering the multitude of microbes that inhabit environmental niches and the multitude of challenges and threats they must confront to survive, it is immediately apparent that these microbes must have individualized survival strategies that rely on the acquisition and maintenance of distinctive attributes. For example, faced with the prospect of dehydration or predation, some microbes have capsules, whereas others encyst and form spores. Environmental fungi, like C. neoformans and H. capsulatum, are vulnerable to predation by protozoa, and each has a unique intracellular survival strategy that enables them to escape being killed by phagocytic cells. Similarly, different microbes employ different strategies to acquire essential nutrients, such as iron. The relative benefit of any one of these attributes is the survival advantage it confers minus its metabolic cost. Based on this calculation, there is no inherently superior strategy if survival is accomplished, with the caveat that some metabolic costs could conceivably reduce fitness. C. neoformans survives in macrophages, and amoebae survive in intracellular polysaccharide-filled vesicles (36), while H. capsulatum survives intracellularly by modulating the phagosomal environment (8, 28). For C. neoformans, capsule synthesis appears to be a metabolically costly process that involves polysaccharide synthesis and secretion (24), while the costs for H. capsulatum to interfere with acidification are unknown but probably lower. Hence, these two intracellular survival strategies are different, yet both accomplish their goal, perhaps at different costs. C. neoformans is found throughout the world, while H. capsulatum is largely geographically restricted to certain locations like the Mississippi-Ohio River valley and to other discrete world locations. The wider distribution of C. neoformans suggests that the factors that govern its environmental survival are more permissive.
In a prior essay, it was proposed that each microbial attribute that allows environmental microbes to survive in the environment could be considered a “card” in a metaphorical game of cards that pits microbes against the environment, with each microbe holding a different card combination in its hand (2). In that formulation, each hand of cards was sufficient for environmental survival, but only some combinations were also suitable for survival and replication in mammalian hosts.
ACCIDENTAL VIRULENCE, CRYPTIC PATHOGENESIS, AND LOST HOSTS
Given that millions of microbial species each potentially holds different “cards” that can potentially function in virulence, a strong case can be made that for some pathogenic microbes, virulence is stochastic, or accidental. In other words, if there are enough microbes with enough different cards, the odds are that some microbes have card combinations that make them virulent for some host. Such a view appears to run counter to the idea that microbial virulence is an intricately complex process selected by microbe-host coevolution. The concept of accidental virulence posits that microbial virulence can arise independently of a host, by chance alone, to generate microbes that possess attributes necessary for virulence in some hosts. Interestingly, this is precisely what H. G. Wells anticipated in his novel, The War of the Worlds, in which a Martian invasion is defeated because certain Earth microbes were pathogenic for the invaders. In that famous science fiction novel, Earth microbes were pathogenic for Martians on their first encounter, without a history of previous host-microbe interaction or coevolution. One might argue that similar host-microbe encounters occurred with human colonization of the Americas or the arrival of Europeans, when numerous animal species that were not indigenous to the new continent were introduced but were found to be susceptible to New World microbes. The ongoing decimation of amphibian populations by a chytrid fungus may reflect a similar phenomenon, whereby ecological changes might select for variants with enhanced virulence (32) or the emergence of new, more-pathogenic variants (25). Genetic recombination may fortuitously lead to new variants with an enhanced ability to occupy a new niche, such as may have caused the emergence of Cryptococcus gattii as a pathogen in North America (14).
An alternative explanation for virulence in microbes acquired from the environment is cryptic pathogenesis, whereby such microbes have animal hosts that are yet to be discovered. In such a scenario, some fraction of the microbial population is always cycling through an animal host, and consequently, attributes for persisting in animal hosts are maintained through selection. The finding that land and marine mammals in areas where Coccidioides immitis is endemic are sometimes found to be infected with this fungus is consistent with a cryptic-pathogenesis explanation (9, 30). Although the possibility of cryptic pathogenesis cannot be excluded for any environmentally acquired microbe, since this would involve proving a negative, there is experimental evidence against an absolute need for animal passage in the maintenance of virulence. Avirulent strains of C. neoformans and H. capsulatum can be restored to virulence through passage in the amoeboid hosts Dictyostelium and Acanthamoeba castellanii, respectively (34, 35).
Yet another explanation for the origin of virulence in environmentally acquired microbes is that they retain ancient traits acquired from hosts that are now extinct. When animals die and decay, any host-associated microbe would presumably have the opportunity to return to the environment. A vast number of animal species that existed in recent epochs are now extinct. For example, horses are a Eurasian mammalian species that is susceptible to New World fungal pathogens, such as C. immitis and H. capsulatum. Since horses did not exist in the Americas prior to the arrival of Europeans, one cannot argue that the virulence of New World fungi in horses is a consequence of the disruption of an established host-microbe relationship. However, it is possible that these fungi retained a capacity for surviving in mammalian hosts from their interactions with now-extinct hosts or other mammals. Support for the latter view comes from the observation of coccidioidomycosis-like lesions in Holocene bison fossils recovered from regions where Coccidioides spp. are currently nonendemic (26). Like cryptic pathogenesis, this explanation remains in the realm of possibility, as it can neither be ruled out nor proven. One could imagine that some traits of environmentally acquired microbes that appear to be ideally suited for animal pathogenesis arose in hosts that are now extinct but were preserved because of dual-use functions that provided survival advantages in the environment.
BENEFITS OF ANIMAL VIRULENCE FOR ENVIRONMENTAL MICROBES
Although the capacity for animal virulence is arguably dispensable for many soil microbes, it is possible that, once acquired, such a capacity confers certain advantages that promote microbial survival in other niches. Occasional passage in animal hosts might increase the fitness of an environmental microbe in its environment. One could imagine situations in which animal passage selects for traits that would confer a survival advantage in soils. For example, animal immune systems could select for variants with adhesins that are suitable for animal host infection, which would in turn provide resistance to soil amoeboid predators. Animal passage could also provide transportation such that soil microbes could spread to new environments. In this regard, it has been proposed that C. immitis reached South America in infected humans and their animals as they migrated from North America (13). Similarly, H. capsulatum may have reached Eurasia through horses infected in the Americas (20). Microbes that can survive in animal hosts can be transported, which is an advantage because they are eventually returned to the soil upon the death and decomposition of the host. Survival in animals implies an ability to survive attack by immune mechanisms, which themselves provide selection pressure that could lead to the emergence of new variants that may be more or less likely to survive in soils when the host dies. In this regard, experimental passage of C. neoformans in mice results in increased virulence, karyotypic changes, phenotypic variants, and antigenic differences (15, 16). When such animal-passaged variants return to the soil, their altered characteristics could provide advantages during confrontations with their natural predators. Hence, the capacity of environmental microbes for survival in animals may arise by chance, but once acquired, it could be maintained because it confers a survival advantage in the environment.
BELIEVERS AND NIHILISTS
The bewildering complexity of microbes, hosts, and their environments can be interpreted to provide fuel for arguments for and against the view that virulence is a special property of only certain microbes. Believers can take heart in the view that virulence is so complex that it can easily be abolished or diminished by altering or removing certain genes. Even among environmentally acquired pathogenic microbes, one can find a delicate relationship between the microbe and host. For example, single mutations in B. dermatitidis that delete its major adhesin can abolish virulence (21). On the other hand, the virulence in animals of certain microbes can be enhanced or restored by passage through amoeboid hosts, suggesting the existence of a more generic quality and that there may not be anything special about pathogenic microbes. While the latter view might foster a sense of nihilism, the complexity of virulence can accommodate both believers and nihilists. It is possible that for some microbes, virulence is indeed a special property that has emerged through the intricate choreography of host-microbe coevolution, while for others, including the microbes that killed the invading Martians in The War of the Worlds, virulence arises by chance in a process that has no explanation, except for that it happened.
Footnotes
Published ahead of print on 19 October 2007.
REFERENCES
- 1.Carmona, E. M., R. Vassallo, Z. Vuk-Pavlovic, J. E. Standing, T. J. Kottom, and A. H. Limper. 2006. Pneumocystis cell wall β-glucans induce dendritic cell costimulatory molecule expression and inflammatory activation through a Fas-Fas ligand mechanism. J. Immunol. 177:459-467. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall, A. 2006. Cards of virulence and the global virulome for humans. Microbe 1:359-364. [Google Scholar]
- 3.Casadevall, A., J. N. Steenbergen, and J. D. Nosanchuk. 2003. ‘Ready made’ virulence and ‘dual-use’ virulence factors in pathogenic environmental fungi—the Cryptococcus neoformans paradigm. Curr. Opin. Microbiol. 6:332-337. [DOI] [PubMed] [Google Scholar]
- 4.Casadevall, A., and L. Pirofski. 2001. Host-pathogen interactions: the attributes of virulence. J. Infect. Dis. 184:337-344. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall, A., and L. A. Pirofski. 2003. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 1:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, Y. C., and K. J. Kwon-Chung. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 14:4912-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards, J. E., Jr. 1991. Invasive candida infections—evolution of a fungal pathogen. N. Engl. J. Med. 324:1060-1062. [DOI] [PubMed] [Google Scholar]
- 8.Eissenberg, L. G., W. E. Goldman, and P. H. Schlesinger. 1993. Histoplasma capsulatum modulates the acidification of phagolysosomes. J. Exp. Med. 177:1605-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emmons, C. W., and A. A. Ashburn. 1942. The isolation of Haplosporangium parvum n. sp. and Coccidioides immitis from wild rodents. Their relationship to coccidioidomycosis. Public Health Rep. 57:1715-2717. [Google Scholar]
- 10.Evans, A. S. 1976. Causation and disease: the Henle-Koch postulates revisited. Yale J. Biol. Med. 49:175-195. [PMC free article] [PubMed] [Google Scholar]
- 11.Falkow, S. 1988. Molecular Koch's postulates applied to microbial pathogenicity. Rev. Infect. Dis. 10(Suppl. 2):S274-S276. [DOI] [PubMed] [Google Scholar]
- 12.Fidel, P. L., Jr. 2005. Immunity in vaginal candidiasis. Curr. Opin. Infect. Dis. 18:107-111. [DOI] [PubMed] [Google Scholar]
- 13.Fisher, M. C., G. L. Koenig, T. J. White, G. San Blas, R. Negroni, I. G. Alvarez, B. Wanke, and J. W. Taylor. 2001. Biogeographic range expansion into South America by Coccidioides immitis mirrors New World patterns of human migration. Proc. Natl. Acad. Sci. USA 98:4558-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser, J. A., S. S. Giles, E. C. Wenink, S. G. Geunes-Boyer, J. R. Wright, S. Diezmann, A. Allen, J. E. Stajich, F. S. Dietrich, J. R. Perfect, and J. Heitman. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360-1364. [DOI] [PubMed] [Google Scholar]
- 15.Fries, B. C., and A. Casadevall. 1998. Serial isolates of Cryptococcus neoformans from patients with AIDS differ in virulence for mice. J. Infect. Dis. 178:1761-1788. [DOI] [PubMed] [Google Scholar]
- 16.Fries, B. C., F. Chen, B. P. Currie, and A. Casadevall. 1996. Karyotype instability in Cryptococcus neoformans infection. J. Clin. Microbiol. 34:1531-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furcolow, M. L. 1961. Airborne histoplasmosis. Bacteriol. Rev. 25:301-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaser, J. B., and A. Garden. 1985. Inoculation of cryptococcosis without transmission of the acquired immunodeficiency syndrome. N. Engl. J. Med. 313:266. [DOI] [PubMed] [Google Scholar]
- 19.Haynes, K. 2001. Virulence in Candida species. Trends Microbiol. 9:591-596. [DOI] [PubMed] [Google Scholar]
- 20.Kasuga, T., T. J. White, G. Koenig, J. McEwen, A. Restrepo, E. Castaneda, L. C. Da Silva, E. M. Heins-Vaccari, R. S. De Freitas, R. M. Zancope-Oliveira, Z. Qin, R. Negroni, D. A. Carter, Y. Mikami, M. Tamura, M. L. Taylor, G. F. Miller, N. Poonwan, and J. W. Taylor. 2003. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol. Ecol. 12:3383-3401. [DOI] [PubMed] [Google Scholar]
- 21.Klein, B. S. 2000. Molecular basis of pathogenicity in Blastomyces dermatitidis: the importance of adhesion. Curr. Opin. Microbiol. 3:339-343. [DOI] [PubMed] [Google Scholar]
- 22.Krause, W., H. Matheis, and K. Wulf. 1969. Fungaemia and funguria after oral administration of Candida albicans. Lancet i:598-599. [DOI] [PubMed] [Google Scholar]
- 23.Lyon, G. M., A. V. Bravo, A. Espino, M. D. Lindsley, R. E. Gutierrez, I. Rodriguez, A. Corella, F. Carrillo, M. M. McNeil, D. W. Warnock, and R. A. Hajjeh. 2004. Histoplasmosis associated with exploring a bat-inhabited cave in Costa Rica, 1998-1999. Am. J. Trop. Med. Hyg. 70:438-442. [PubMed] [Google Scholar]
- 24.Maxson, M. E., E. Cook, A. Casadevall, and O. Zaragoza. 2007. The volume and hydration of the Cryptococcus neoformans polysaccharide capsule. Fungal Genet. Biol. 44:180-186. [DOI] [PubMed] [Google Scholar]
- 25.Morgan, J. A., V. T. Vredenburg, L. J. Rachowicz, R. A. Knapp, M. J. Stice, T. Tunstall, R. E. Bingham, J. M. Parker, J. E. Longcore, C. Moritz, C. J. Briggs, and J. W. Taylor. 2007. Population genetics of the frog-killing fungus Batrachochytrium dendrobatidis. Proc. Natl. Acad. Sci. USA 104:13845-13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrow, W. 2006. Holocene coccidioidomycosis: Valley Fever in early Holocene bison (Bison antiquus). Mycologia 98:669-677. [DOI] [PubMed] [Google Scholar]
- 27.Muir, R., and M.-W. Tan. 2006. Evolution of pathogens in soil, p. 131-146. In H. S. Seifert and V. J. DiRita (ed.), Evolution of microbial pathogens. ASM Press, Washington, DC.
- 28.Newman, S. L., L. Gootee, J. Hilty, and R. E. Morris. 2006. Human macrophages do not require phagosome acidification to mediate fungistatic/fungicidal activity against Histoplasma capsulatum. J. Immunol. 176:1806-1813. [DOI] [PubMed] [Google Scholar]
- 29.Nosanchuk, J. D., and A. Casadevall. 2006. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 50:3519-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappagianis, D. 1998. Coccidioides immitis, p. 357-371. In L. Ajello and R. J. Hay (ed.), Topley & Wilson's microbiology and microbial infections. Arnold, London, United Kingdom.
- 31.Petersen, L. R., S. L. Marshall, C. Barton-Dickson, R. A. Hajjeh, M. D. Lindsley, D. W. Warnock, A. A. Panackal, J. B. Shaffer, M. B. Haddad, F. S. Fisher, D. T. Dennis, and J. Morgan. 2004. Coccidioidomycosis among workers at an archeological site, northeastern Utah. Emerg. Infect. Dis. 10:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pounds, J. A., M. R. Bustamante, L. A. Coloma, J. A. Consuegra, M. P. Fogden, P. N. Foster, M. E. La, K. L. Masters, A. Merino-Viteri, R. Puschendorf, S. R. Ron, G. A. Sanchez-Azofeifa, C. J. Still, and B. E. Young. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439:161-167. [DOI] [PubMed] [Google Scholar]
- 33.Rappleye, C. A., and W. E. Goldman. 2006. Defining virulence genes in the dimorphic fungi. Annu. Rev. Microbiol. 60:281-303. [DOI] [PubMed] [Google Scholar]
- 34.Steenbergen, J. N., J. D. Nosanchuk, S. D. Malliaris, and A. Casadevall. 2003. Cryptococcus neoformans virulence is enhanced after growth in the genetically malleable host Dictyostelium discoideum. Infect. Immun. 71:4862-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steenbergen, J. N., J. D. Nosanchuk, S. D. Malliaris, and A. Casadevall. 2004. Interaction of Blastomyces dermatitidis, Sporothrix schenckii, and Histoplasma capsulatum with Acanthamoeba castellanii. Infect. Immun. 72:3478-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 18:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor, M. L., C. B. Chavez-Tapia, A. Rojas-Martinez, M. del Rocio Reyes-Montes, M. B. del Valle, and G. Zuniga. 2005. Geographical distribution of genetic polymorphism of the pathogen Histoplasma capsulatum isolated from infected bats, captured in a central zone of Mexico. FEMS Immunol. Med. Microbiol. 45:451-458. [DOI] [PubMed] [Google Scholar]
- 38.Tekaia, F., and J. P. Latge. 2005. Aspergillus fumigatus: saprophyte or pathogen? Curr. Opin. Microbiol. 8:385-392. [DOI] [PubMed] [Google Scholar]
- 39.Thomas, C. F., Jr., and A. H. Limper. 2007. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat. Rev. Microbiol. 5:298-308. [DOI] [PubMed] [Google Scholar]
- 40.von Eiff, C., G. Peters, and C. Heilmann. 2002. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect. Dis. 2:677-685. [DOI] [PubMed] [Google Scholar]
- 41.Winiecka-Krusnell, J., and E. Linder. 1999. Free-living amoebae protecting Legionella in water: the tip of an iceberg? Scand. J. Infect. Dis. 31:383-385. [DOI] [PubMed] [Google Scholar]
- 42.Yang, Y. L. 2003. Virulence factors of Candida species. J. Microbiol. Immunol. Infect. 36:223-228. [PubMed] [Google Scholar]