Abstract
Challenge with the intracellular protozoan parasite Toxoplasma gondii induces a potent CD8+ T-cell response that is required for resistance to infection, but many questions remain about the factors that regulate the presentation of major histocompatibility complex class I (MHC-I)-restricted parasite antigens and about the role of professional and nonprofessional accessory cells. In order to address these issues, transgenic parasites expressing ovalbumin (OVA), reagents that track OVA/MHC-I presentation, and OVA-specific CD8+ T cells were exploited to compare the abilities of different infected cell types to stimulate CD8+ T cells and to define the factors that contribute to antigen processing. These studies reveal that a variety of infected cell types, including hematopoietic and nonhematopoietic cells, are capable of activating an OVA-specific CD8+ T-cell hybridoma, and that this phenomenon is dependent on the transporter associated with antigen processing and requires live T. gondii. Several experimental approaches indicate that T-cell activation is a consequence of direct presentation by infected host cells rather than cross-presentation. Surprisingly, nonprofessional antigen-presenting cells (APCs) were at least as efficient as dendritic cells at activating this MHC-I-restricted response. Studies to assess whether these cells are involved in initiation of the CD8+ T-cell response to T. gondii in vivo show that chimeric mice expressing MHC-I only in nonhematopoietic compartments are able to activate OVA-specific CD8+ T cells upon challenge. These findings associate nonprofessional APCs with the initial activation of CD8+ T cells during toxoplasmosis.
Accessory cells process microbial antigens and present antigen-derived peptides to the immune system in the context of major histocompatibility complex class I (MHC-I). This process is essential for the activation of CD8+ T cells that is required for long-term resistance to many intracellular pathogens. The canonical MHC-I antigen-processing pathway involves presentation of self peptides and foreign peptides from the cytosol and allows CD8+ T lymphocytes to directly recognize infected cells. In addition, an alternative pathway, in which professional antigen-presenting cells (APCs) such as dendritic cells (DCs) sample the environment and process exogenous antigens for cross-presentation to CD8+ T cells to initiate a specific response, has been described (3, 11, 30, 53). The latter pathway is thought to be involved in the activation of naïve CD8+ T cells following infection with various viruses (1, 30, 39, 53), intracellular bacteria (24, 30, 65), and the protozoan parasite Plasmodium yoelii (24).
Infection of normal hosts with Toxoplasma gondii elicits a Th1-type response, characterized by the generation of parasite-specific CD4+ and CD8+ T cells producing gamma interferon (IFN-γ) to provide protective immunity (13). Although numerous reports detail an important role for CD8+ T cells in resistance to acute, chronic, and memory responses to T. gondii (5, 14, 26, 40, 54-56), our understanding of how this pathogen primes CD8+ T-cell responses remains incomplete, as infection with T. gondii poses several challenges to the priming of CD8+ T-cell responses. For example, parasite infection of DCs and macrophages (MΦ) appears to interfere with multiple signaling cascades involved in the maturation of DC populations that are required for the development of protective immunity (12, 19, 32, 47, 50). In addition, in the infected cell, T. gondii multiplies within a nondegradative parasitophorous vacuole (PV), and this compartment has until recently been viewed as inaccessible to the host endocytic machinery required for MHC-I presentation of endogenous antigens (52). It is also interesting that this pathogen is capable of replicating within any nonhematopoietic cell type; whether these infected cells contribute to the priming of T-cell responses has not been explored. Thus, despite evidence of a considerable infection-induced activation of CD8+ T cells, there remain many questions about the mechanisms that underlie how these lymphocytes become primed and recognize infected cells (33).
Major obstacles to a more-detailed mechanistic understanding of the events leading to T. gondii-induced activation of CD8+ T cells include difficulties in imaging antigen presentation and the inability to track clonotypic T-cell responses with certainty (18). Such problems have been overcome for various bacterial and parasitic systems through the use of model antigens for which reagents are available to track T-cell activation and antigen presentation (2, 16, 41, 42, 65). Similar strategies have been applied to T. gondii (28), including work relevant to CD8+ T-cell responses, in which DCs from mice infected with a virulent, ovalbumin (OVA)-expressing strain of T. gondii require transporter associated with antigen processing 1 (TAP1) to activate a CD8+ T-cell hybridoma expressing a β-galactosidase reporter (B3Z cells) (20). While those studies did not distinguish between productively infected cells and those containing dead parasites, or those which may have acquired parasite-derived material from the environment, they are consistent with a model in which T. gondii infection leads to OVA presentation via the endogenous MHC-I pathway.
Most models of CD8+ T-cell priming envision professional APCs (particularly DCs) as required for antigen presentation, as they are able to provide the requisite second signals necessary for a productive T-cell response. However, this view has been challenged in studies where infected nonhematopoietic cells reach the lymphoid environment (27, 30). In order to elucidate the factors that regulate CD8+ T-cell responses to T. gondii, we have employed transgenic parasites secreting a truncated form of OVA (41) to compare the abilities of different cell types to present antigen and also to investigate the host factors that influence these events. These studies reveal that T. gondii antigens are presented by actively infected cells, rather than bystander cells, using the endogenous MHC-I pathway to activate CD8+ T cells. Studies conducted in vitro and in vivo implicate nonhematopoietic cells as playing an important role in the regulation of the CD8+ T-cell response.
MATERIALS AND METHODS
T. gondii culture and transgenic parasite lines.
Parental and transgenic T. gondii strains Prugniaud ΔHXGPRT (kindly provided by D. Soldati, University of Geneva, Geneva, Switzerland) and RHΔcpsII (where cps indicates carbamoyl phosphate synthase; kindly provided by D. Bzik, Dartmouth College, NH) were maintained as tachyzoites by serial passage in human foreskin fibroblast (HFF) cell monolayers as previously described (45). To allow RHΔcpsII parasite growth, tissue culture medium was supplemented with 0.2 mM uracil. For mouse infections, parasites were purified by filtration through a 3.0-μm filter (Nuclepore; Steriltech Corp., Kent, WA) and washed with medium or phosphate-buffered saline (PBS).
Transgenic Prugniaud ΔHXGPRT parasites were engineered to secrete a truncated form of OVA (amino acids 140 to 386) into the PV (via the dense granule pathway) as previously described (41). In order to generate similar uracil auxotrophs, RHΔcpsII parasites were transfected with the plasmid ptubP30-OVA/sagCAT by electroporation, selected in the presence of 20 μM chloramphenicol, and cloned by limiting dilution (41, 45).
Preparation of APCs: DCs, MΦ, astrocytes, and fibroblasts.
DCs were grown from mouse bone marrow (BM) in the presence of granulocyte-macrophage colony-stimulating factor as previously described (34, 36); purity was >85% based on CD11c expression. MΦ were also grown from mouse BM using 30% L-cell-conditioned medium (from L929 cells) as previously described (7). Primary astrocytes were harvested from the brain frontal lobes of 1- to 3-day-old mice as previously described (62); purity was >90% based on GFAP (glial fibrillary acidic protein) expression. MC3T3-E1 subclone 30 fibroblast (C57BL/6, ATCC CRL-2596) cell monolayers were cultivated in Alpha minimum essential medium with ribonucleosides and desoxyribonucleosides but without ascorbic acid (Invitrogen Corp., Carlsbad. CA), supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, and 10% fetal bovine serum. NIH 3T3 fibroblasts were grown in Dulbecco's modified Eagle's medium (Gibco) as described for HFF cell monolayers (45). All cells were cultivated at 37°C in 5% CO2 in a humidified atmosphere.
In vitro OVA/MHC-I presentation.
OVA presentation in the context of H-2Kb was assessed in vitro by using the B3Z CD8+ T-cell hybridoma (kindly provided by N. Shastri, University of California, Berkeley, CA) (25) as previously described (20, 25, 51). BM-DCs or BM-MΦ (105) or astrocytes or fibroblasts (5 × 104) were inoculated into flat-bottom 96-well plates and incubated for 4 to 6 h (BM-DCs) or overnight (other cell types). When primed, cells were cultivated in the presence of 100 U/ml recombinant murine IFN-γ and 10 U/ml tumor necrosis factor alpha (TNF-α) for 4 h, followed by the addition of 10 μl of antigen or parasites (0.1 μg/ml OVA amino acid 257 to 264 [OVA257-264] peptide SIINFEKL [Mr, 1,088; 92 pM; Invitrogen], 500 μg/ml soluble OVA [Worthington, Lakewood, NJ], live T. gondii tachyzoites at a multiplicity of infection [MOI] of 0.5, or heat-killed tachyzoites [treated 20 min at 56°C] at an MOI of 5) and incubation for an additional 16 h. Plates were spun and washed twice in RPMI medium without phenol red (Gibco), and 105 B3Z CD8+ T cells were added to 100 μl of RPMI (supplemented with 10% fetal bovine serum, 2 mM glutamine, 1 mM sodium pyruvate, 0.5 μM 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin; without phenol red) and incubated for 16 h. β-Galactosidase activity was detected by adding chlorophenol red-β-d-galactopyranoside (CPRG; Calbiochem, San Diego, CA) at a final concentration of 100 μM for 16 h, and absorbance was measured at 562 nm. B3Z cell activation was expressed as the increase (n-fold) in absorbance, relative to values obtained in the absence of antigen or parasites. Supernatants from B3Z cell assays were also tested for interleukin-2 secretion by enzyme-linked immunosorbent assay, and yielding results were consistent with optical density (OD) readings for B3Z cell activation (data not shown).
Flow cytometry and reagents.
Single-cell suspensions were generated from the spleens or mesenteric lymph nodes of mice at 8 days postinfection or from naïve mice, washed, and incubated with Fc Block (BD Pharmingen, San Jose, CA) for 15 min on ice. Cells were then analyzed directly ex vivo for proliferation using carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Invitrogen, Carlsbad, CA) and for expression of surface markers using the following monoclonal antibodies (MAbs): fluorescein-conjugated anti-H-2Kb, phycoerythrin-conjugated anti-CD11b or -CD8α, peridin chlorophyll a protein (PerCP)-conjugated anti-CD8α, or allophycocyanin-conjugated anti-CD11c, -CD62L, or -Thy1.1 (or corresponding isotype controls). After 30 min on ice, samples were washed twice and fixed in 4% (wt/vol) paraformaldehyde (PFA), and data were acquired with a FACSCalibur flow cytometer using CellQuest software (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Tree Star, Inc., Ashland, OR).
Immunofluorescence assays and microscopy.
Immunolabeling of OVA was conducted with tachyzoite-infected HFF cell monolayers grown on 22-mm round glass coverslips. Cells were fixed in 4% (wt/vol) PFA, permeabilized in 0.2% (vol/vol) Triton X-100 in PBS, incubated in blocking buffer (10% fetal bovine serum and 0.1% Triton X-100 in PBS) for 1 h, and labeled with rabbit anti-OVA polyclonal antiserum (Bethyl, Inc., Montgomery, TX) diluted 1:1,000 and Alexa Fluor 488-conjugated goat anti-rabbit antibody (Molecular Probes) diluted 1:2,000 in blocking buffer. Fluorescence was detected with a Zeiss Axiovert 35 microscope equipped with a 100-W Hg vapor lamp, appropriate barrier-emission filters, and an interline transfer chip charge-coupled-device camera (Hamamatsu). Images were captured, colored, and contrast adjusted by using Openlab software (Improvision, Lexington, MA).
OVA presentation in the context of MHC-I was assessed with primary astrocytes grown on 12-mm round glass coverslips, primed with IFN-γ and TNF-α, and cultivated for 24 h with either 5 μg/ml SIINFEKL peptide, heat-killed Prugniaud ΔHXGPRT secreted Ova (SecOVA), or live Prugniaud ΔHXGPRT SecOVA. Cells were washed for 15 min in fresh medium at 37°C and incubated for 30 min at 37°C with MAb 25D1.16, which is specific for the SIINFEKL/MHC-I complex (kindly provided by R. Germain, National Institute of Allergy and Infectious Diseases, Bethesda, MD) (43). Monolayers were directly fixed in 4% PFA and washed extensively in PBS before staining with Alexa Fluor 488-conjugated goat anti-mouse antibody as described above; DNA was stained with 4′,6′-diamidino-2-phenylindole (DAPI; Molecular Probes). Fluorescence was detected using an Olympus IX-70 inverted microscope equipped with an Hg arc bulb and appropriate barrier-emission filters, coupled to an Applied Precision DeltaVision deconvolution system (Applied Precision, LLC, Issaquah, WA). Z sections were captured and deconvolved with SoftWoRx software (Applied Precision).
Mice, adoptive cell transfer, and T. gondii infection.
Female C57BL/6, BALB/c, B6.129S2-Tap1tm1Arp/J, and B6.129P2-B2 mtm1Unc/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). OT-1 Thy1.1 (expressing a transgenic T-cell receptor designed to recognize OVA257-264 [SIINFEKL] in the context of H-2Kb) and C57BL/6 Thy1.2 mice were originally obtained from H. Shen and P. Scott, respectively, and bred within the University Laboratory Animal Resources facility of the University of Pennsylvania. All mice were maintained under specific-pathogen-free conditions in accordance with institutional guidelines. Intraperitoneal inoculation with T. gondii was performed by injection with 104 Prugniaud ΔHXGPRT or Prugniaud ΔHXGPRT SecOVA tachyzoites in 0.1 ml PBS.
For adoptive transfers, naïve CD3+ T cells were purified from the spleen of wild-type or OT-1 Thy1.1 mice after erythrocyte depletion with 0.86% NH4CL (Sigma-Aldrich, St. Louis, MO), using CD3+-specific columns (R&D Systems, Minneapolis, MN). C57BL/6 Thy1.2 mice received 2 × 106 cells, labeled with CFSE to assess proliferation, in 0.2 ml PBS by retro-orbital injection. Intraperitoneal infection was performed 1 day posttransfer by using 104 tachyzoites in 0.1 ml PBS.
Generation of chimeric mice.
BM was isolated from the femur and tibia of male B6.129P2-B2 mtm1Unc/J mice (Jackson Laboratory), processed to generate single-cell suspensions, and treated with ACK lysis buffer (BioWhittaker, Walkersville, MD) to deplete erythrocytes. After filtration to remove large particles, T cells were depleted by using MACS CD90 (Thy1.2) MicroBeads (Miltenyi Biotec, Auburn, CA). Male C57BL/6 mice designated for BM transplantation were pretreated 4 days with neomycin irrigant (Neosporin GU, 40 mg base, and polymyxin B sulfate, 200,000 U; Monarch Pharmaceuticals, Bristol, TN) by adding 1 ml/liter of sterile drinking water (replaced every 2 days). Eighteen hours after 1,000-rad irradiation, 3 × 106 cells of the B6.129P2-B2 m−/− mouse marrow preparation were injected into anesthetized mice via the dorsal vein of the penis. Recipients were maintained on the neomycin regimen for an additional 14 days and used for experiments at least 10 weeks posttransplant. Chimeric mice were adoptively transferred with 2 × 106 CFSE-labeled naïve CD3+ T cells or OT-I T cells in 0.2 ml PBS by retro-orbital injection and infected 1 day later with 104 tachyzoites in 0.1 ml PBS.
RESULTS
Infection of both hematopoietic and nonhematopoietic cells leads to antigen presentation.
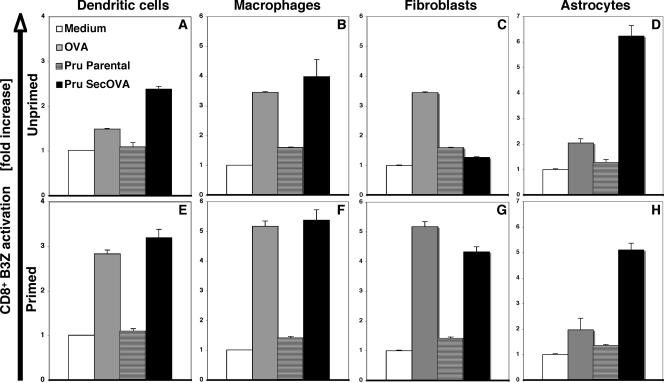
DCs, MΦ, fibroblasts, and astrocytes have all been implicated in the protective immune response to T. gondii (13, 22, 64). In order to directly compare the ability of various primed and unprimed cell types to process and present MHC-I-restricted T. gondii antigens, we employed an in vitro antigen presentation assay (20, 25, 51). Transgenic cyst-forming Prugniaud parasites were engineered to secrete OVA (41), and infected cells were then assayed for their ability to activate an OVA-specific, MHC-I-restricted B3Z T-cell hybridoma. Infection of all unprimed cell types with live parental (Fig. 1) or heat-killed Prugniaud ΔHXGPRT-OVA (not shown) parasites failed to stimulate the B3Z cell line (compare with results for uninfected controls). Substantial activation was observed when unprimed MΦ or fibroblasts (but not DCs or astrocytes) were pulsed with OVA protein, (Fig. 1, top), but Prugniaud ΔHXGPRT SecOVA parasites produced a different pattern, inducing unprimed DCs, MΦ, and astrocytes (but not fibroblasts) to activate the B3Z cell response (Fig. 1, top).
FIG. 1.
MHC-I presentation of OVA by hematopoietic and nonhematopoietic cells infected with OVA transgenic T. gondii. DCs, MΦ, fibroblasts, or astrocytes were cultivated without (top panels) or with (bottom panels) IFN-γ and TNF-α, followed by 16 h of incubation with 500 μg/ml OVA protein, 5 × 104 Prugniaud ΔHXGPRT strain (Pru) T. gondii tachyzoites, or 5 × 104 Prugniaud ΔHXGPRT strain T. gondii engineered to express SecOVA. Professional APCs were prepared from the BM of C57BL/6 mouse (H-2Kb) MHC-I, and OVA presentation was assayed using a B3Z CD8+ T-cell hybridoma that specifically expresses LacZ upon interaction with MHC-I (Kb)/OVA257-264 (SIINFEKL) complexes (25). Results (averages for at least five experiments with triplicate samples ± standard deviation [SD]) are expressed as the increase (n-fold) in the OD at 562 nm (OD562) relative to samples where antigen (but not B3Z cells) was omitted (white bars). In contrast to professional APCs, fibroblasts require priming in order to present SecOVA to B3Z cells. At the time when OD562 values are read for B3Z cell activation (48 h postinfection), parasites have replicated but have not lysed out from the originally infected APCs; if assays were left until host cell lysis and reinfection of other cells (including T cells) occurred, then the negative-control Prugniaud ΔHXGPRT parental strain samples (striped bars) would become positive (wells would turn from yellow to purple).
Given the important role of IFN-γ and TNF-α in the regulation of multiple genes, including those involved in antigen presentation (4, 29), and the requirement of these factors for resistance to T. gondii, the ability of these cytokines to influence T-cell activation was assessed. Priming of DCs and MΦ led to increased levels of T-cell activation in response to either OVA or Prugniaud ΔHXGPRT SecOVA parasites; in addition, priming stimulated a strong T-cell response in fibroblasts (Fig. 1, lower panels). As controls, BM-DCs that were generated from β2-microglobulin knock-out mice (which lack appreciable surface MHC-I) did not induce any B3Z cell activation, whether or not they were IFN-γ/TNF-α primed (data not shown). Together, these data show that infection with live parasites secreting OVA into the PV leads to B3Z CD8+ cell activation and that DCs, MΦ, and astrocytes do not require priming to present T. gondii SecOVA to the B3Z CD8+ T cells, but treatment with IFN-γ and TNF-α is required for fibroblasts to activate the hybridoma.
Levels of MHC-I presentation correlate with the ability to present antigen.
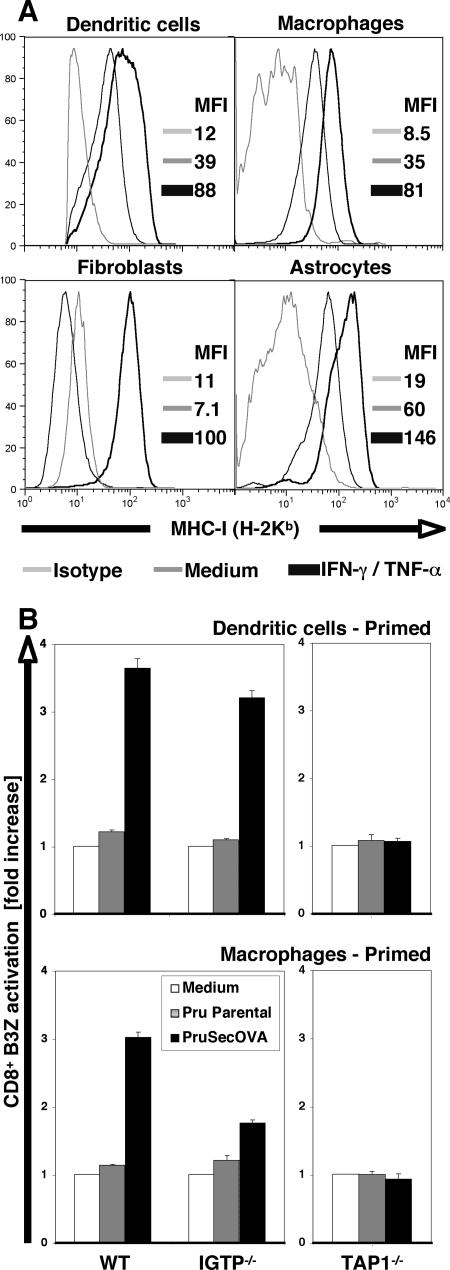
The studies presented above highlight inherent differences in the ability of diverse cell types to present parasite-derived antigens and demonstrate that priming with IFN-γ/TNF-α can enhance antigen presentation. One possible explanation for these observations is that cell types may differ in their expression of MHC-I (which influences T-cell activation) and that MHC expression is upregulated by treatment with IFN-γ. Surface staining for H-2Kb by flow cytometry supports this hypothesis, as constitutive MHC-I expression was detected for DCs, MΦ, and astrocytes, and these levels were enhanced by IFN-γ treatment; significant MHC-I staining was detectable for fibroblasts only when these cells were primed (Fig. 2A). These data are consistent with one of the major properties of IFN-γ.
FIG. 2.
The relationship between IFN-γ and MHC-I presentation of SecOVA. (A) Cell surface expression of MHC-I (H-2Kb) molecules in unprimed or IFN-γ/TNF-α primed BM-DCs, BM-MΦ, fibroblasts, and astrocytes, determined by flow cytometry. MFI, mean fluorescence intensity. Note the strong induction in primed nonhematopoietic cells. This is consistent with the hypothesis that, in fibroblasts, priming (i) is required to activate SecOVA presentation via the endogenous MHC-I pathway and (ii) enhances the presentation of exogenous OVA via cross-presentation (Fig. 1C and 1G). In DCs, this activation of the endogenous MHC-I pathway is not required for SecOVA presentation, but it does enhance both endogenous and cross-presentation pathways (Fig. 1A and 1E). (B) BM-DCs (top) and BM-MΦ (bottom) from IGTP- or TAP1-deficient mice were primed with IFN-γ/TNF-α and assessed for the ability to present SecOVA to B3Z cells in vitro. Results are expressed as increases (n-fold) relative to results for controls incubated without antigen (white bars). WT, wild type.
In addition to its ability to enhance MHC-I expression, IFN-γ is also known to induce various antimicrobial effector mechanisms that inhibit T. gondii growth (63), some of which may play a role in facilitating the entry of parasite-derived proteins into the host cytosol and the MHC-I presentation pathway. The IFN-γ-induced p47 GTPase (IGTP) is required for MΦ control of parasite replication and has recently been implicated in the breakdown of the PVs in T. gondii-infected cells (6, 22, 57). Using cells prepared from IGTP−/− animals, we found little reduction in the ability of IFN-γ/TNF-α-primed DCs infected with Prugniaud ΔHXGPRT SecOVA parasites to activate the B3Z cells, but IGTP-deficient MΦ-mediated activation was significantly reduced, although not completely abolished (Fig. 2B, left panels). Nitric oxide has also been implicated as an important molecule for control of T. gondii (17, 31), but NG-monomethyl-l-arginine, which inhibits inducible nitric oxide synthase, did not affect the ability of primed or unprimed MΦ and DCs to activate the B3Z hybridoma (not shown).
Transfer of cytosolic peptides into the endoplasmic reticulum is mediated by TAP, enabling assembly with MHC-I molecules; this process has also been implicated in some cases of cross-presentation (38). When DCs and MΦ generated from TAP1-deficient mice were used in the in vitro antigen presentation assay, no B3Z cell activation was observed (Fig. 2B, right panels), consistent with previous reports on TAP-dependent SecOVA transport into the host cell endoplasmic reticulum (2, 20).
Direct MHC-I presentation by infected cells.
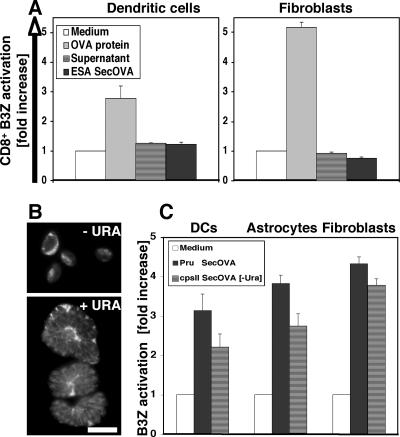
The studies described above do not distinguish between the processing and presentation of antigen directly within infected cells themselves and the cross-presentation of antigen acquired from the environment (e.g., through endocytosis of parasites or material from cells lysed during the course of infection). Several strategies were used to address this question. First, concentrated parasite culture supernatants (from fully lysed infected cells or excreted/secreted antigens from extracellular tachyzoites) (9) were tested for their ability to activate B3Z cells (Fig. 3A). In contrast to the activation observed when IFN-γ/TNF-α-primed DCs or fibroblasts were incubated with exogenous soluble OVA or with infected cells (Fig. 1 and 2B), neither of the SecOVA-containing supernatants activated the B3Z hybridoma.
FIG. 3.
MHC-I presentation of SecOVA from intracellular parasites versus lysed cell/parasite supernatant. (A) IFN-γ/TNF-α-primed H-2Kb BM-DCs and fibroblasts were incubated for 16 h with either OVA protein (500 μg/ml), concentrated supernatant from a lysed-out culture of ∼5 × 107 Prugniaud ΔHXGPRT SecOVA parasites, or excreted/secreted antigens from 5 × 104 Prugniaud ΔHXGPRT SecOVA tachyzoites, and MHC-I/OVA presentation was measured using the B3Z cell assay; the number of parasites used corresponds to conditions employed in antigen presentation assays. B3Z cells were activated only when APCs were incubated with large amounts of exogenous soluble OVA (shown are averages for three experiments performed in triplicate ± SD). (B) RHΔcpsII T. gondii parasites (15) engineered to secrete OVA into the PV were incubated with or without 0.2 mM uracil (URA) prior to staining with polyclonal anti-OVA. In these uracil auxotrophs, replication (and therefore cytolysis) is dependent on uracil, but parasites remain alive for several days, continuing to metabolize and secrete OVA protein into the PV. Bar = 10 μm. (C) IFN-γ/TNF-α-primed H-2Kb BM-DCs, astrocytes, and fibroblasts were incubated for 16 h with 5 × 104 wild-type Prugniaud ΔHXGPRT (Pru) SecOVA parasites or 5 × 104 nonreplicating RHΔcpsII SecOVA parasites in the absence of uracil (−Ura). MHC-I presentation of SecOVA (detected using the B3Z cell assay) did not require parasite replication or host cell cytolysis, suggesting an endogenous origin of the presented antigen (average results from five or more experiments performed in triplicate ± SD).
In a second approach to address the possibility that parasite-mediated lysis of the host cells (releasing PV contents) could allow cross-presentation of SecOVA from the medium, RHΔcpsII knock-out parasites were engineered to express secOVA (Fig. 3B). These mutants are unable to synthesize pyrimidines de novo (15); they invade host cells normally and secrete their dense granule contents (including SecOVA) into the PV (top), but cannot replicate without the addition of exogenous uracil (bottom). As shown in Fig. 3C, T. gondii SecOVA-infected, IFN-γ/TNF-α-primed DCs, astrocytes, and fibroblasts were all able to induce B3Z cell activation without parasite replication and consequent host cell lysis. The higher levels of B3Z cell activation generated by infection with replicating Prugniaud ΔHXGPRT SecOVA parasites is probably attributable to the larger number of parasites, and hence SecOVA, produced. Thus, neither T. gondii replication nor host cell lysis is required for MHC-I antigen presentation of parasite-derived antigens to B3Z CD8+ T cells.
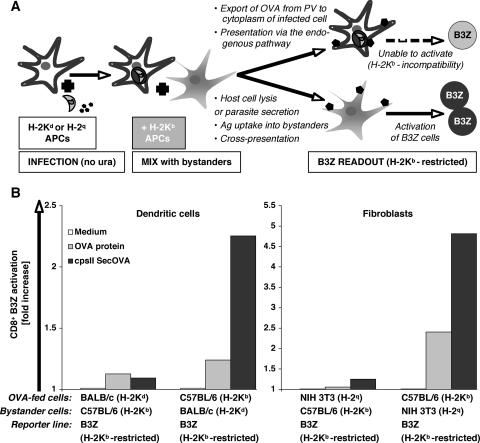
Haplotype mixing was also exploited to test cross-presentation versus endogenous antigen presentation in T. gondii-infected cultures, as diagrammed in Fig. 4A. IFN-γ- and TNF-α-primed DCs from BALB/c mice (H-2Kd), or NIH 3T3 fibroblasts (H-2q haplotype), were infected with RHΔcpsII SecOVA parasites for 12 h. After extensive washes, primed DCs or fibroblasts from C57BL/6 mice (H-2Kb) were added for another 12 h before the addition of B3Z cells. Cultures were carried out in the absence of uracil to avoid parasite replication and host cell lysis. The inclusion of C57BL/6 APCs would indicate whether activation of the B3Z hybridoma (reactive against SIINFEKL when presented by the H-2Kb haplotype only) is attributable to the T. gondii SecOVA-infected cells themselves (in which case activation will not occur) or bystander cells (presumably due to antigen release, uptake by the C57BL/6 APCs, and cross-presentation). As shown in Fig. 4B, B3Z T-cell activation was observed only when H-Kb cells, but not H-2Kd or H-2q APCs, were infected. This result indicates that cross-presentation via bystander cells was not involved in the activation of the CD8+ T cells in this experimental system.
FIG. 4.
Actively infected cells, rather than bystanders, present T. gondii SecOVA in the context of MHC-I. (A) Experimental design for a haplotype-mixing experiment based on the restricted reactivity of B3Z CD8+ T cells to H-2Kb MHC-I molecules. First, 105 IFN-γ/TNF-α-primed APCs bearing haplotypes H-2Kd (BALB/c BM-DCs) or H-2q (NIH 3T3 fibroblasts) are incubated with OVA protein (500 μg/ml) or infected with RHΔcpsII SecOVA parasites (MOI = 0.5) and incubated for 12 h to permit invasion. These cells are then mixed with 105 APCs bearing the H-2Kb haplotype, which are capable of activating B3Z cells (reacting with the H-2Kb haplotype). In theory, lysis of the infected host cell or antigen secretion in the medium, followed by antigen uptake by bystander APCs, could activate B3Z cells via cross-presentation (lower pathway). Infected (or OVA-treated) cells are unable to activate B3Z cells by either the endogenous pathway or lysis and cross-presentation, due to H-2K incompatibility. (B) When APCs expressing H-2Kd (BALB/c BM-DC) or H-2q (NIH 3T3 cell) haplotypes were exposed to antigen, no cross-presentation was observed in vitro. If washes were omitted from the protocol before the addition of bystander cells, B3Z cell activation occurred, because H-2Kb APCs were then in direct contact with antigens or parasites (not shown). As the control, H-2Kb BM-DCs or fibroblasts were exposed to antigen (OVA peptide or SecOVA parasites), resulting in strong activation (average from three independent experiments). cpsII, RHΔcpsII.
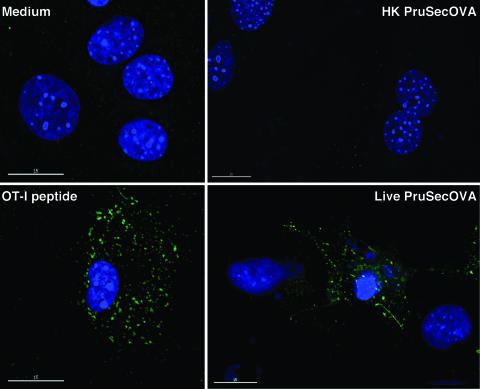
Finally, the MAb 25D1.16, which is specific for the MHC-I/SIINFEKL complex (43), was used to directly visualize expression of this complex. Because astrocytes are particularly well suited to morphological analysis, cell monolayers were incubated with live or heat-killed Prugniaud ΔHXGPRT SecOVA parasites, fixed, permeabilized, and stained with DAPI to visualize DNA. As shown in Fig. 5, the 25D1.16 MAb did not label cells in uninfected cultures (top left), staining only a few cells that had ingested heat-killed Prugniaud ΔHXGPRT SecOVA parasites (top right). In contrast, intense, discrete MHC-I/OVA staining was observed with cells incubated with OT-I peptide or infected with live tachyzoites of Prugniaud ΔHXGPRT SecOVA (bottom panels). Taken together, these data do not formally exclude cross-presentation by bystander cells, but are consistent with a model in which actively infected host cells do process and present T. gondii SecOVA via the endogenous MHC-I pathway.
FIG. 5.
MHC-I presentation of SecOVA only in host cells actively infected with T. gondii transgenics. Visualization of MHC-I/SIINFEKL complexes in primed astrocyte cultures that were untreated (top left), incubated with the SIINFEKL OT-I peptide (bottom left), exposed to heat-killed Prugniaud ΔHXGPRT SecOVA (PruSecOVA) parasites (top right), or actively infected with live Prugniaud ΔHXGPRT SecOVA parasites (bottom right). Green staining indicates MHC-I/SIINFEKL complexes detected using MAb 25D1.16 (43); blue indicates DAPI staining of host and parasite DNA. Note the intense staining of MHC-I/SIINFEKL complexes in cells that contain several PVs with replicating Prugniaud ΔHXGPRT SecOVA parasites. These clusters are still unidentified and would be MHC-I/SIINFEFL complexes either at the cell surface (concentrated in specific areas) and/or in the exocytic/endocytic pathways, because incubation with the 25D1.16 MAb was carried out using live cells, and MHC/peptide complexes may be internalized during this incubation. Uninfected (bystander) cells were not labeled.
Activation of an OVA-specific CD8+ T-cell response by transgenic T. gondii in vivo.
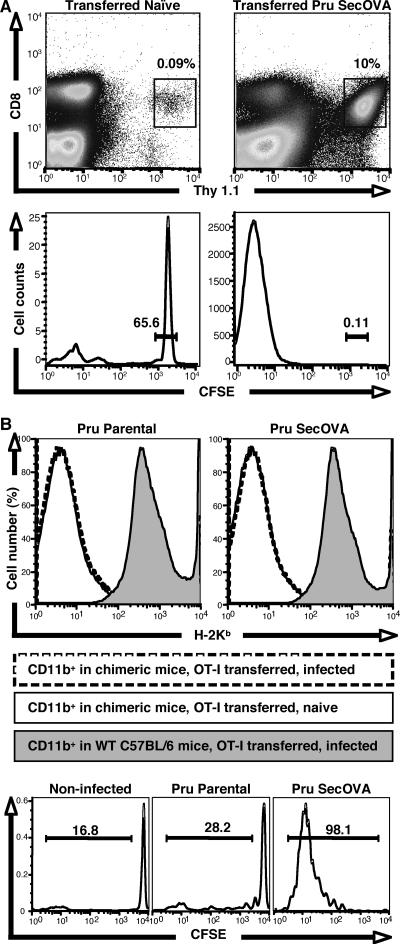
While in vitro studies using the B3Z hybridoma provide a good surrogate measure for CD8+ T-cell activation and demonstrate that nonhematopoietic cells can be involved in antigen presentation, it was unclear if these events actually take place during a natural infection in vivo. In order to first determine whether infection of mice with Prugniaud ΔHXGPRT SecOVA T. gondii induces a CD8+ T-cell response, we carried out a series of adoptive transfer experiments similar to those using OVA-specific DO11 CD4+ T cells as previously described (41). B6 Thy1.2 mice were transferred with naïve CFSE-labeled OVA-specific transgenic OT-I Thy1.1 T cells, followed by infection with Prugniaud ΔHXGPRT parental or Prugniaud ΔHXGPRT SecOVA parasites. Mesenteric lymph nodes were collected 8 days postinfection, and cell proliferation was analyzed by gating on the CD3+CD8+ Thy1.1 population and assessing CFSE dilution. In transferred naïve mice, OT-I Thy1.1 T cells constituted <0.1% of the total CD8+ T-cell population (Fig. 6A, top left), and these cells remained CFSEhigh (bottom left), consistent with previous reports (41). Infection with parental Prugniaud ΔHXGPRT parasites did not increase OT-I T-cell numbers or affect CFSE levels (not shown). In mice infected with Prugniaud ΔHXGPRT SecOVA parasites, however, OVA-specific T cells proliferated and accounted for ∼10% of the total CD8+ T-cell population (top right), diluting CFSE levels (bottom right). Thus, the transgenic SecOVA parasites stimulate a specific CD8+ T-cell response in vivo, which can be detected using adoptively transferred OT-I T cells.
FIG. 6.
Expansion and activation of CD8+ T cells in mice infected with T. gondii expressing SecOVA. (A) MHC-I/SIINFEKL-restricted OT-I Thy1.1 CD8+ T cells were labeled with CFSE and adoptively transferred to C57BL/6 Thy1.2 mice. Eight days after infection with Prugniaud ΔHXGPRT (Pru) SecOVA parasites (right panels) or mock treatment (left panels), cells in the draining mesenteric lymph nodes were stained for CD3, CD8, and Thy1.1 (top), and the positive populations (boxed) were assessed for retention of CFSE (bottom). Far more OVA-specific CD8+ T cells were observed in Prugniaud ΔHXGPRT SecOVA-infected animals than in naïve controls, and dilution of CFSE confirms proliferation of these cells. (B) Irradiated wild-type C57BL/6 mice were reconstituted with BM from β2-microglobulin-deficient animals, producing chimeras expressing MHC-I in nonhematopoietic tissues only. Chimeric mice were then infected with Prugniaud ΔHXGPRT parental or Prugniaud ΔHXGPRT SecOVA parasites 1 day after adoptive transfer of CFSE-labeled OT-I T cells. H-2Kb surface staining assayed by flow cytometry of the CD11b+ population from the mesenteric lymph nodes (top panels) shows minimal levels of MHC-I expression in the chimeric mice (no shading) relative to that seen with the wild-type C57BL/6 controls (shaded), regardless of infection status (solid versus broken lines). Nevertheless, CFSE dilution shows proliferation of the CD8+ OT-I T-cell population in the mesenteric lymph nodes 8 days after infection with Prugniaud ΔHXGPRT SecOVA (lower right), but not in uninfected (left) or Prugniaud ΔHXGPRT parental strain-infected animals (center).
Role for nonhematopoietic cells in regulation of the CD8+ T-cell response.
Nonhematopoietic cells are generally viewed as unable to prime naïve CD8+ T cells, but the observation that fibroblasts and astrocytes are potent activators of the CD8+ B3Z hybridoma (Fig. 1 to 5) suggests that these nonclassical APCs may in fact play a role in initiating lymphocyte responses. In order to test the contribution of this pathway to CD8+ T-cell activation during T. gondii infection in vivo, C57BL/6 mice were sublethally irradiated and reconstituted with BM from β2-microglobulin-deficient mice to produce chimeric animals in which surface MHC-I is expressed only in nonhematopoietic compartments (see Materials and Methods). MHC-I expression was low in the CD11b+ population obtained from mesenteric lymph nodes of chimeric mice (mostly MΦ, with some DCs and B cells), regardless of infection status (Fig. 6B, top), whereas fibroblasts from these mice expressed significant levels of MHC-I (not shown). To assay infection-induced CD8+ T-cell activation, CFSE-labeled OT-I CD8+ T cells were transferred into chimeras, followed by infection the next day with either parental or SecOVA-expressing parasites. Reciprocal chimeric mice were not generated, because such mice would have no endogenous CD8+ T cells and transferred OTI CD8+ T cells would homeostatically expand. Mesenteric lymph nodes were collected 8 days postinfection and assessed for OTI CD8+ T-cell proliferation. An OVA-specific CD8+ T-cell response was detected for chimeric mice infected with Prugniaud ΔHXGPRT SecOVA (Fig. 6B, bottom right) but not for animals infected with the parental Prugniaud ΔHXGPRT strain or for uninfected controls. These data raise the possibility that nonhematopoietic cells may play a role in the priming and expansion of CD8+ T cells during toxoplasmosis.
DISCUSSION
It has long been appreciated that infection with T. gondii induces a strong MHC-I restricted-CD8+ T-cell response and that these cells are required for the control of parasite replication (5, 14, 26, 40, 54-56). Several challenges remain in understanding the development of this cellular response, however, including the nature of the unique intracellular environment (the PV) within which parasites reside (52), how this compartment interacts with the host cell, and how parasite infection interferes with host signaling pathways (12, 19, 32, 47, 50). Although the PV is distinct from endocytic pathway compartments, and despite parasite-mediated interference with immune activation, there is evidence that T. gondii antigens may be cross-presented to CD8+ T cells (14, 21). Antigen presentation could derive from the endocytosis or phagocytosis of antigens secreted by extracellular parasites or released during the lysis of infected cells. However, the in vitro assays presented in Fig. 1 to 5 indicate that infected cells can stimulate the MHC-I-restricted response directly and that this occurs via the endogenous MHC-I pathway.
The MHC-I-restricted presentation of SecOVA by infected cells and the detection of the MHC-I/SIINFEKL complex in infected astrocytes (Fig. 5) imply that parasite proteins (or peptides) can translocate from the PV into the host cell cytoplasm or that infected cells are somehow able to sample the PV (6, 22, 35, 57, 58). During intracellular parasite development, PV maturation is thought to be driven by the secretion of dense granule and rhoptry proteins (23, 37). The PV membrane acts as a sieve, allowing the influx of nutrients and other small molecules, of <1,300 to 1,900 Da (48), but this size exclusion could still potentially allow antigenic peptides from the PV to enter the host cell cytoplasm (for example, the octapeptide SIINFEKL is ∼1,000 Da). Elegant studies employing a sensitive Cre recombinase assay demonstrated the release of larger proteins from the PV in at least some infected cells (20), and we have also detected OVA protein by immunofluorescence in a small percentage of fibroblasts infected by Prugniaud ΔHXGPRT SecOVA parasites (<1%). Several reports demonstrate the trafficking of specific rhoptry proteins from the PV into the host cell cytoplasm (19, 46, 47, 59), although the mechanism whereby proteins cross the PV membrane remains unknown. A recently demonstrated mode of nutrient acquisition, involving the recruitment of host cell microtubules that provoke membranous invaginations and appear to deliver host lysosomes to the PV (10), could also play a role in compromising the PV barrier. The evidence that host cells may exploit specific mechanisms to sample from the PV is more limited. The observation that IGTP plays a role in antigen presentation by MΦ but not DCs (Fig. 2) is intriguing and suggests that there may be a lineage-specific role for this GTPase in antigen presentation and that related protein families (LRG47, IRG47, TGTP, IIGP) may be important in different cell types.
Perhaps the most unexpected finding from this study is the observation that nonclassical APCs are as capable as DCs and MΦ in activating B3Z cells. T. gondii invades a variety of nonhematopoietic cells, and chimeric approaches have revealed that these cells are required for resistance to both acute and chronic infections, functioning as inducible nitric oxide synthase-independent mediators of IFN-γ- and TNF-α-dependent immunity (8, 22, 64). Current dogma holds that nonhematopoietic cells are unable to prime naïve CD8+ T cells, but the abilities of fibroblasts and astrocytes to activate the B3Z hybridoma in vitro (Fig. 1 to 4), and the data from β2-microglobulin radiation chimeras (Fig. 6), suggest a role for this mechanism of CD8+ T-cell activation. Although previous studies are consistent with a model in which IFN-γ and TNF-α activate infected nonhematopoietic cells to directly inhibit parasite replication, it may be that priming of nonhematopoietic cells by these cytokines is required for the development of CD8+ T-cell responses and long-term resistance to T. gondii. Several studies indicate that nonprofessional APCs can play a role in the development of resistance to other intracellular pathogens. For example, a specific CD8+ T-cell response against lymphocytic choriomeningitis virus (LCMV) can be primed by fibroblasts expressing LCMV antigens that reach lymphoid organs (27), a response that can develop independently of BM-derived APCs (30). These studies implicate the cytokine microenvironment as critical, and it is therefore notable that T. gondii generates inflammation in lymphoid organs and elicits a strong CD8+ T-cell response. The suggestion that the expression of MHC-I by hematopoietic cells may not be required for CD8+ T-cell proliferation raises several questions about the signals required for CD8+ T-cell activation. In particular, since T. gondii-induced expansion of CD8+ T cells is largely CD28 independent (44, 61), and nonprofessional accessory cells lack classical B7 costimulatory molecules, CD8+ T-cell function during T. gondii or LCMV infection is likely to require the involvement of other costimulatory pathways in the non-BM-derived APCs (30, 44, 49, 60). DCs and MΦ clearly play an important role in the sampling and presentation of microbial antigens and in providing costimulatory and cytokine-mediated signals that influence the magnitude and polarity of T-cell responses, but the ability of diverse host cells to present pathogen-derived antigens to CD8+ T cells may diminish the requirement for priming within secondary lymphoid organs. Whether this is sufficient for the development of a fully functional protective response (whether the T cells are cytolytic, produce normal levels of IFN-γ, can persist, form memory, or traffic appropriately) is the subject of ongoing studies.
Acknowledgments
We thank David Bzik, Ronald Germain, Philip Scott, Nilabh Shastri, Hao Shen, and Dominique Soldati for providing valuable reagents and Qun Fang, Tamika Seals, and Sapna Suravajjala for expert technical assistance.
This work was supported by National Institutes of Health grants AI028724 to D.S.R.; AI42334, State of Pennsylvania, to C.A.H.; and AI062789 to L.T. D.S.R. is an Ellison Medical Foundation Senior Scholar in Global Infectious Diseases.
The authors have no financial conflicts of interest.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 10 September 2007.
REFERENCES
- 1.Arrode, G., C. Boccaccio, J. Lule, S. Allart, N. Moinard, J.-P. Abastado, A. Alam, and C. Davrinche. 2000. Incoming human cytomegalovirus pp65 (UL83) contained in apoptotic infected fibroblasts is cross-presented to CD8+ T cells by dendritic cells. J. Virol. 74:10018-10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertholet, S., R. Goldszmid, A. Morrot, A. Debrabant, F. Afrin, C. Collazo-Custodio, M. Houde, M. Desjardins, A. Sher, and D. Sacks. 2006. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J. Immunol. 177:3525-3533. [DOI] [PubMed] [Google Scholar]
- 3.Bevan, M. J. 1976. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp. Med. 143:1283-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 5.Brown, C. R., C. A. Hunter, R. G. Estes, E. Beckmann, J. Forman, C. David, J. S. Remington, and R. McLeod. 1995. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology 85:419-428. [PMC free article] [PubMed] [Google Scholar]
- 6.Butcher, B. A., R. I. Greene, S. C. Henry, K. L. Annecharico, J. B. Weinberg, E. Y. Denkers, A. Sher, and G. A. Taylor. 2005. p47 GTPases regulate Toxoplasma gondii survival in activated macrophages. Infect. Immun. 73:3278-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caamano, J., J. Alexander, L. Craig, R. Bravo, and C. A. Hunter. 1999. The NF-kappa B family member RelB is required for innate and adaptive immunity to Toxoplasma gondii. J. Immunol. 163:4453-4461. [PubMed] [Google Scholar]
- 8.Collazo, C. M., G. S. Yap, S. Hieny, P. Caspar, C. G. Feng, G. A. Taylor, and A. Sher. 2002. The function of gamma interferon-inducible GTP-binding protein IGTP in host resistance to Toxoplasma gondii is Stat1 dependent and requires expression in both hematopoietic and nonhematopoietic cellular compartments. Infect. Immun. 70:6933-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppens, I., M. Andries, J. L. Liu, and M. F. Cesbron-Delauw. 1999. Intracellular trafficking of dense granule proteins in Toxoplasma gondii and experimental evidences for a regulated exocytosis. Eur. J. Cell Biol. 78:463-472. [DOI] [PubMed] [Google Scholar]
- 10.Coppens, I., J. D. Dunn, J. D. Romano, M. Pypaert, H. Zhang, J. C. Boothroyd, and K. A. Joiner. 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125:261-274. [DOI] [PubMed] [Google Scholar]
- 11.Cresswell, P., A. L. Ackerman, A. Giodini, D. R. Peaper, and P. A. Wearsch. 2005. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol. Rev. 207:145-157. [DOI] [PubMed] [Google Scholar]
- 12.Denkers, E. Y., and B. A. Butcher. 2005. Sabotage and exploitation in macrophages parasitized by intracellular protozoans. Trends Parasitol. 21:35-41. [DOI] [PubMed] [Google Scholar]
- 13.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denkers, E. Y., R. T. Gazzinelli, S. Hieny, P. Caspar, and A. Sher. 1993. Bone marrow macrophages process exogenous Toxoplasma gondii polypeptides for recognition by parasite-specific cytolytic T lymphocytes. J. Immunol. 150:517-526. [PubMed] [Google Scholar]
- 15.Fox, B. A., and D. J. Bzik. 2002. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 415:926-929. [DOI] [PubMed] [Google Scholar]
- 16.Garg, N., M. P. Nunes, and R. L. Tarleton. 1997. Delivery by Trypanosoma cruzi of proteins into the MHC class I antigen processing and presentation pathway. J. Immunol. 158:3293-3302. [PubMed] [Google Scholar]
- 17.Gazzinelli, R. T., S. Hayashi, M. Wysocka, L. Carrera, R. Kuhn, W. Muller, F. Roberge, G. Trinchieri, and A. Sher. 1994. Role of IL-12 in the initiation of cell mediated immunity by Toxoplasma gondii and its regulation by IL-10 and nitric oxide. J. Eukaryot. Microbiol. 41:9S. [PubMed] [Google Scholar]
- 18.Germain, R. N., and M. K. Jenkins. 2004. In vivo antigen presentation. Curr. Opin. Immunol. 16:120-125. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert, L. A., S. Ravindran, J. M. Turetzky, J. C. Boothroyd, and P. J. Bradley. 2007. Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot. Cell 6:73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gubbels, M. J., B. Striepen, N. Shastri, M. Turkoz, and E. A. Robey. 2005. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect. Immun. 73:703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakim, F. T., R. T. Gazzinelli, E. Denkers, S. Hieny, G. M. Shearer, and A. Sher. 1991. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J. Immunol. 147:2310-2316. [PubMed] [Google Scholar]
- 22.Halonen, S. K., G. A. Taylor, and L. M. Weiss. 2001. Gamma interferon-induced inhibition of Toxoplasma gondii in astrocytes is mediated by IGTP. Infect. Immun. 69:5573-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joiner, K. A., and D. S. Roos. 2002. Secretory traffic in the eukaryotic parasite Toxoplasma gondii: less is more. J. Cell Biol. 157:557-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2002. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karttunen, J., S. Sanderson, and N. Shastri. 1992. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. USA 89:6020-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasper, L. H., I. A. Khan, K. H. Ely, R. Buelow, and J. C. Boothroyd. 1992. Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against Toxoplasma gondii-infected peritoneal macrophages. J. Immunol. 148:1493-1498. [PubMed] [Google Scholar]
- 27.Kundig, T. M., M. F. Bachmann, C. DiPaolo, J. J. Simard, M. Battegay, H. Lother, A. Gessner, K. Kuhlcke, P. S. Ohashi, H. Hengartner, et al. 1995. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science 268:1343-1347. [DOI] [PubMed] [Google Scholar]
- 28.Kwok, L. Y., S. Lutjen, S. Soltek, D. Soldati, D. Busch, M. Deckert, and D. Schluter. 2003. The induction and kinetics of antigen-specific CD8 T cells are defined by the stage specificity and compartmentalization of the antigen in murine toxoplasmosis. J. Immunol. 170:1949-1957. [DOI] [PubMed] [Google Scholar]
- 29.Langermans, J. A., M. E. Van der Hulst, P. H. Nibbering, P. S. Hiemstra, L. Fransen, and R. Van Furth. 1992. IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J. Immunol. 148:568-574. [PubMed] [Google Scholar]
- 30.Lenz, L. L., E. A. Butz, and M. J. Bevan. 2000. Requirements for bone marrow-derived antigen-presenting cells in priming cytotoxic T cell responses to intracellular pathogens. J. Exp. Med. 192:1135-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luder, C. G., M. Algner, C. Lang, N. Bleicher, and U. Gross. 2003. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int. J. Parasitol. 33:833-844. [DOI] [PubMed] [Google Scholar]
- 32.Luder, C. G., C. Lang, M. Giraldo-Velasquez, M. Algner, J. Gerdes, and U. Gross. 2003. Toxoplasma gondii inhibits MHC class II expression in neural antigen-presenting cells by down-regulating the class II transactivator CIITA. J. Neuroimmunol. 134:12-24. [DOI] [PubMed] [Google Scholar]
- 33.Luder, C. G., and F. Seeber. 2001. Toxoplasma gondii and MHC-restricted antigen presentation: on degradation, transport and modulation. Int. J. Parasitol. 31:1355-1369. [DOI] [PubMed] [Google Scholar]
- 34.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 35.Martens, S., I. Parvanova, J. Zerrahn, G. Griffiths, G. Schell, G. Reichmann, and J. C. Howard. 2005. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathogens 1:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKee, A. S., F. Dzierszinski, M. Boes, D. S. Roos, and E. J. Pearce. 2004. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J. Immunol. 173:2632-2640. [DOI] [PubMed] [Google Scholar]
- 37.Mercier, C., K. D. Adjogble, W. Daubener, and M. F. Delauw. 2005. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? Int. J. Parasitol. 35:829-849. [DOI] [PubMed] [Google Scholar]
- 38.Norbury, C. C., S. Basta, K. B. Donohue, D. C. Tscharke, M. F. Princiotta, P. Berglund, J. Gibbs, J. R. Bennink, and J. W. Yewdell. 2004. CD8+ T cell cross-priming via transfer of proteasome substrates. Science 304:1318-1321. [DOI] [PubMed] [Google Scholar]
- 39.Norbury, C. C., D. Malide, J. S. Gibbs, J. R. Bennink, and J. W. Yewdell. 2002. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 3:265-271. [DOI] [PubMed] [Google Scholar]
- 40.Parker, S. J., C. W. Roberts, and J. Alexander. 1991. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin. Exp. Immunol. 84:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pepper, M., F. Dzierszinski, A. Crawford, C. A. Hunter, and D. Roos. 2004. Development of a system to study CD4+-T-cell responses to transgenic ovalbumin-expressing Toxoplasma gondii during toxoplasmosis. Infect. Immun. 72:7240-7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pope, C., S. K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402-3409. [DOI] [PubMed] [Google Scholar]
- 43.Porgador, A., J. W. Yewdell, Y. Deng, J. R. Bennink, and R. N. Germain. 1997. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6:715-726. [DOI] [PubMed] [Google Scholar]
- 44.Reichmann, G., E. N. Villegas, L. Craig, R. Peach, and C. A. Hunter. 1999. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J. Immunol. 163:3354-3362. [PubMed] [Google Scholar]
- 45.Roos, D. S., R. G. Donald, N. S. Morrissette, and A. L. Moulton. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45:27-63. [DOI] [PubMed] [Google Scholar]
- 46.Saeij, J. P., J. P. Boyle, S. Coller, S. Taylor, L. D. Sibley, E. T. Brooke-Powell, J. W. Ajioka, and J. C. Boothroyd. 2006. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314:1780-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saeij, J. P., S. Coller, J. P. Boyle, M. E. Jerome, M. W. White, and J. C. Boothroyd. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445:324-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwab, J. C., C. J. Beckers, and K. A. Joiner. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. USA 91:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261:609-612. [DOI] [PubMed] [Google Scholar]
- 50.Shapira, S., O. S. Harb, J. Caamano, and C. A. Hunter. 2004. The NF-kappaB signaling pathway: immune evasion and immunoregulation during toxoplasmosis. Int. J. Parasitol. 34:393-400. [DOI] [PubMed] [Google Scholar]
- 51.Shastri, N., and F. Gonzalez. 1993. Endogenous generation and presentation of the ovalbumin peptide/Kb complex to T cells. J. Immunol. 150:2724-2736. [PubMed] [Google Scholar]
- 52.Sibley, L. D. 2003. Toxoplasma gondii: perfecting an intracellular life style. Traffic 4:581-586. [DOI] [PubMed] [Google Scholar]
- 53.Sigal, L. J., and K. L. Rock. 2000. Bone marrow-derived antigen-presenting cells are required for the generation of cytotoxic T lymphocyte responses to viruses and use transporter associated with antigen presentation (TAP)-dependent and -independent pathways of antigen presentation. J. Exp. Med. 192:1143-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subauste, C. S., A. H. Koniaris, and J. S. Remington. 1991. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J. Immunol. 147:3955-3959. [PubMed] [Google Scholar]
- 55.Suzuki, Y., K. Joh, M. A. Orellana, F. K. Conley, and J. S. Remington. 1991. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology 74:732-739. [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki, Y., and J. S. Remington. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943-3946. [PubMed] [Google Scholar]
- 57.Taylor, G. A., C. M. Collazo, G. S. Yap, K. Nguyen, T. A. Gregorio, L. S. Taylor, B. Eagleson, L. Secrest, E. A. Southon, S. W. Reid, L. Tessarollo, M. Bray, D. W. McVicar, K. L. Komschlies, H. A. Young, C. A. Biron, A. Sher, and G. F. Vande Woude. 2000. Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc. Natl. Acad. Sci. USA 97:751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor, G. A., C. G. Feng, and A. Sher. 2004. p47 GTPases: regulators of immunity to intracellular pathogens. Nat. Rev. Immunol. 4:100-109. [DOI] [PubMed] [Google Scholar]
- 59.Taylor, S., A. Barragan, C. Su, B. Fux, S. J. Fentress, K. Tang, W. L. Beatty, H. E. Hajj, M. Jerome, M. S. Behnke, M. White, J. C. Wootton, and L. D. Sibley. 2006. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314:1776-1780. [DOI] [PubMed] [Google Scholar]
- 60.Villegas, E. N., M. M. Elloso, G. Reichmann, R. Peach, and C. A. Hunter. 1999. Role of CD28 in the generation of effector and memory responses required for resistance to Toxoplasma gondii. J. Immunol. 163:3344-3353. [PubMed] [Google Scholar]
- 61.Villegas, E. N., L. A. Lieberman, N. Mason, S. L. Blass, V. P. Zediak, R. Peach, T. Horan, S. Yoshinaga, and C. A. Hunter. 2002. A role for inducible costimulator protein in the CD28-independent mechanism of resistance to Toxoplasma gondii. J. Immunol. 169:937-943. [DOI] [PubMed] [Google Scholar]
- 62.Wilson, E. H., U. Wille-Reece, F. Dzierszinski, and C. A. Hunter. 2005. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J. Neuroimmunol. 165:63-74. [DOI] [PubMed] [Google Scholar]
- 63.Yap, G. S., M. H. Shaw, Y. Ling, and A. Sher. 2006. Genetic analysis of host resistance to intracellular pathogens: lessons from studies of Toxoplasma gondii infection. Microbes Infect. 8:1174-1178. [DOI] [PubMed] [Google Scholar]
- 64.Yap, G. S., and A. Sher. 1999. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 189:1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yrlid, U., M. Svensson, C. Johansson, and M. J. Wick. 2000. Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response. FEMS Immunol. Med. Microbiol. 27:313-320. [DOI] [PubMed] [Google Scholar]