Abstract
Lipopolysaccharide (LPS) is a major component of the outer membrane of gram-negative bacteria, and the lipid A region of LPS mediates stimulation of the immune system in a structure-dependent manner. Unlike the LPS of many other gram-negative bacteria, the LPS of Francisella tularensis isolated from in vitro cultures is not proinflammatory. This observed lack of proinflammatory prowess may reflect structural features of the lipid A, such as the number and length of the acyl chains and the single-phosphate group. To better understand this phenotype, we have begun to elucidate LPS biosynthesis in F. tularensis. We present complementation, mutational, and chemical data demonstrating that F. tularensis FTT0232c encodes a functional late acyltransferase enzyme with specificity similar to that of the Escherichia coli LpxL ortholog. Expression of this late acyltransferase complemented the temperature-sensitive and hypoacylated lipid A phenotypes of an E. coli lpxL mutant, expression of FTT0232c is increased during intracellular growth relative to that during in vitro growth, and finally, LPS obtained from a mutant of F. tularensis lacking FTT0232c showed an abundant triacyl lipid A species after mass spectrometric analysis, consistent with the loss of an LpxL late acyltransferase.
Francisella tularensis is a gram-negative, nonsporulating, encapsulated, facultative intracellular coccobacillus that causes tularemia, a zoonotic disease of humans and other mammals. Tularemia is an acute febrile illness that commonly causes local ulceration at the site of entry (20). F. tularensis is transmitted to humans and other mammals by the bite of infected insects or animals, by direct inoculation of the mucosa, by ingestion of contaminated water or food, and, most seriously, by inhalation of aerosolized bacteria (34, 44). Inhalation of as few as 10 bacteria can cause serious disease in humans (46). Because of its high infectivity, ease of aerosol dissemination, and severe morbidity and mortality, F. tularensis is considered a possible biological warfare agent (19). Although the natural reservoir of F. tularensis is undetermined, F. tularensis survives and replicates within the protozoan Acanthamoebae castellanii in aquatic environments and persists in mud and water for more than 1 year (1, 35).
Our understanding of F. tularensis pathogenesis is incomplete. During mammalian infection, it is generally believed that the macrophage is the major reservoir of F. tularensis. In vitro, fresh serum containing active complement enhances bacterial uptake by human monocyte-derived macrophages (MDM) and human monocyte-like THP-1 cells (12, 13). Phagocytosis of opsonized bacteria occurs via interaction with complement receptor 3 and the extension of large, asymmetric pseudopod loops from the macrophage surface (12). Once inside the phagosome, living F. tularensis resides within a phagosome that transiently associates with early endosomes and then accumulates markers of the late endosomal pathway (1, 13, 45). Although it acquires late endosomal markers, the Francisella-containing phagosome does not mature further and only moderately undergoes acidification (13, 45). After 3 to 4 h of phagocytosis, disruption of the phagosomal membrane occurs, corresponding with the loss of lamp-1 and CD63 and bacterial escape into the host cell cytosol (1, 13). Because all F. tularensis isolates studied escape the phagosome and replicate within the cell cytoplasm, a conserved virulence determinant(s) likely is responsible. Transposon mutagenesis of F. tularensis identified two genes, iglB and iglC, essential for intramacrophage growth (24). In addition, mutation of mglA or mglB, encoding transcriptional regulators of genes located within the Francisella pathogenicity island, inhibits phagosomal escape, intramacrophage growth, and virulence in mice (5, 24).
The lipopolysaccharide (LPS) of F. tularensis is structurally and biologically distinct from the LPS of other gram-negative bacteria. The lipid A moiety of F. tularensis LPS is asymmetrically tetra-acylated with atypically long acyl chains (16 and 18 carbons), lacks a 4′ phosphate residue, and is replaced at the 1 position of the diglucosamine molecule with a phosphogalactosamine (36, 53). Likely a result of the lipid A structure, F. tularensis LPS fails to stimulate cytokine release from mononuclear cells or upregulate surface immunoglobins on B cells (36, 43, 53). In contrast, Escherichia coli LPS is hexaacylated and phosphorylated at the 1 and 4′ positions (39). E. coli LPS complexes with myeloid differentiation protein 2 to interact with Toll-like receptor 4 (TLR4) on macrophages and endothelial cells and functions as a proinflammatory endotoxin, eliciting the release of inflammation mediators, such as tumor necrosis factor-α (TNF-α) and interleukin-1β, and costimulatory molecules required for the adaptive immune response (41).
Biological activity of lipid A is affected by the degree of acylation, and the most potent form of lipid A is hexaacylated. In E. coli, late acyltransferase enzymes LpxL and LpxM sequentially add the fifth and sixth fatty acids, respectively, to precursor lipid IVA (tetra-acyl lipid A) after glycosylation with two 3-deoxy-d-manno-octulosonic acid (Kdo) sugars (14, 48). In E. coli, laurate transferase, encoded by lpxL (formerly htrB), acylates (Kdo)2-lipid IVA, adding a lauric acid (C12:0) to 3-hydroxymyristic acid [C14:0(3-OH)] at the 2′ position of the distal glucosamine (14). E. coli lpxL mutant bacteria cease dividing above 33°C, are deformed in rich medium, and eventually lose viability (26). E. coli myristate transferase, encoded by lpxM (formerly msbB), uses the penta-acylated lipid A structure as a substrate, adding a myristic acid (C14:0) onto 3-hydroxymyristic acid [C14:0(3-OH)], which is located at the 3′ position of the distal glucosamine (15).
We report the identification and function of LpxL, a late acyltransferase enzyme encoded by FTT0232c, in F. tularensis. lpxL was identified by searching the F. tularensis subsp. tularensis strain SCHU S4 genome database with E. coli LpxL. Overexpression of F. tularensis lpxL in E. coli MLK217, a strain that synthesizes tetra-acylated lipid A when grown at 37°C because of a mutation in the lpxL gene, resulted in hexa-acylated lipid A and in bacteria able to grow at 42°C. Moreover, an F. tularensis FTT0232c mutant was constructed that produced a major triacyl lipid A species lacking a single fatty acid residue, confirming its role as a late acyltransferase.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. E. coli strains were grown in Luria-Bertani (LB) medium with appropriate antibiotics at 30, 37, or 42°C with agitation at 225 rpm. For E. coli strains, antibiotics were used at the following concentrations: 125 μg/ml ampicillin, 15 μg/ml tetracycline, 50 μg/ml streptomycin, 50 μg/ml kanamycin, and 20 μg/ml chloramphenicol. F. tularensis subsp. holarctica strain 1547-57 (referred to as F. tularensis in this work) was isolated from a patient with cutaneous tularemia at the University of Iowa Hospitals and Clinics. F. tularensis was grown on chocolate agar medium (Remel, Lenexa, KS), and the F. tularensis live vaccine strain (LVS) was grown on cystine heart agar medium (Difco Co., Sparks, MD) containing 9% defibrinated sheep's blood (Colorado Serum Co., Denver, CO) (CHAB) at 32, 37, or 42°C with 5% CO2 for 24 to 96 h. Liquid cultures of Francisella were grown in Mueller-Hinton broth (Difco Co.) supplemented with 0.1% glucose, 0.025% ferric pyrophosphate, and 2% Isovitalex and were agitated at 150 rpm at 37°C with 5% CO2 for 24 to 48 h. Spectinomycin (50 μg/ml) and 50 μg/ml kanamycin were used during Francisella culture, as appropriate. All work involving F. tularensis strain 1547-57 was carried out in a Centers for Disease Control and Prevention-approved biosafety level 3 laboratory with select agent approval.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype, relevant phenotype, and/or selection markera | Source or reference |
|---|---|---|
| Strains | ||
| E. coli TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara leu)7697 galU galK rpsL (Strr) endIA1 nupG | Invitrogen |
| E. coli B178 | W3110 galE sup+ | 12 |
| E. coli MLK217 | B178 lpxL::mini Tn10, Tetr | 12 |
| E. coli MKM10 | MLK217, pMKM.11, Apr, Tetr | This study |
| E. coli MKM11 | MLK217, pMKM.21, Apr, Tetr | This study |
| E. coli MLK1067 | B178 lpxM::Ωcam | 14 |
| E. coli MKM13 | MLK1067, pMKM.11, Apr, Cmr | This study |
| F. tularensis subsp. holarctica strain 1547-57 | Wild-type, human isolate | 36 |
| F. tularensis MKM87 | Strain 1547-57, lpxL::aphA-3, Kmr | This study |
| Plasmids | ||
| pBluescript II SK(−) | Apr, cloning vector | Stratagene |
| pCR2.1TOPO | Apr, Kmr, cloning vector | Invitrogen |
| pCRspec2 | pCR2.1TOPO, aad(9), Apr, Kmr, Spr | This study |
| pFNLTP10grosacB | pFNLTP9 derivative, LVS groEL promoter, sacB, temperature-sensitive origin of Francisella replication, Kmr, Apr | Gift from T. Zhart |
| pMKM.1 | F. tularensis FTT0232c in pCR2.1, Apr, Kmr | This study |
| pMKM.11 | pBluescript II SK(−), EcoRI fragment from pMKM.1 containing FTT0232c, Kmr | This study |
| pMKM.16 | pCR2.1TOPO, FTT0232c and flanking sequence without consensus sequence regions, Apr, Kmr | This study |
| pMKM.17 | pMKM.16, aphA-3, Apr, Kmr | This study |
| pMKM.2 | pCR2.1TOPO, FTT0231c, Apr, Kmr | This study |
| pMKM.21 | pBluescript II SK(−), EcoRI fragment from pMKM.1 containing FTT0231c, Kmr | This study |
| pMKM.219c | 7.5-kb plasmid containing F. tularensis temperature-sensitive origin of replication, LVS groEL promoter upstream of sacB, E. coli origin of replication, aad(9), Spr | This study |
| pMKM.223 | pMKM.219c, FTT0232c::aphA-3, Spr, Kmr | This study |
| pSPECR | pCRBlunt (Invitrogen), aad(9), Spr | 57 |
| pUC18K | aphA-3, Kmr | 33 |
Spr, Apr, and Kmr, resistance to spectinomycin, ampicillin, and kanamycin, respectively.
DNA manipulations.
Restriction and modifying enzymes were purchased from New England Biolabs (Beverly, MA). Standard recombinant DNA procedures were performed as described previously (42). PCR was performed using the Taq polymerase PCR kit (QIAGEN, Valencia, CA) using standard reaction conditions and oligonucleotides listed in Table 2 (42). PCR products were cloned into pCR2.1TOPO using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA). T4 DNA ligase (New England BioLabs) was used for all other cloning. Transformation was performed using One Shot Top10 competent E. coli cells (Invitrogen) or MAX Efficiency DH5α competent E. coli cells (Invitrogen). Plasmids were isolated from bacteria using the Qiaprep Spin mini prep kit (QIAGEN). Sequence analysis was performed by The University of Iowa DNA Sequencing Core Facility using an ABI 3700 sequencer. Genomic DNA was isolated from F. tularensis using the Puregene genomic DNA purification kit (Gentra Systems, Minneapolis, MN).
TABLE 2.
Oligonucleotides used in this studya
| Name and procedure | Nucleotide sequence (5′-3′) | Gene or target |
|---|---|---|
| PCR | ||
| MKM1 | CCAGTGCTAATGGGATTATCAAT | FTT0232C |
| MKM4 | GGTTCTCATAAGCGATATCATTA | FTT0232C |
| MKM5 | GGTAATGATCTAGAAGATGCGAA | FTT0231C |
| MKM6 | GGTATCTGGATTGTCGTTGGTTA | FTT0232C |
| MKM7 | GGTCTGGTTCTATAGCGACGAT | FTT0232C |
| MKM8 | GCCGCACTGGCTGCTGAA | FTT0231C |
| MKM82 | GTACCCCGGGGGAGCATACCACATAGAGAC | FTT0232C |
| MKM83 | CATGCCCGGGGGTATCTGGATTGTCGTTGG | FTT0232C |
| MKM85 | CAGTGGATCCGGCTGTTTGCCTATGCTTATACA | FTT0232C |
| MKM86 | CTGAGGATCCGGTATACCTATAGATGCAAGAATA | FTT0232C |
| MKM104b | GCACTCGACTGCAGGGATCTTCTTGAGATCCTTT | oriE |
| MKM105 | CCGAGCTACTCGAGTATACTTTGGCGTCACCCCT | sacB |
| MKM106 | GACTCTCGAGCTGCACGACGAACTAATACT | groEL promoter |
| MKM107 | CGTCCTCGAGCCGATTTAGAGCTTGACGG | F. tularensis ori |
| MKM128 | AGAATATCACCGGAATTGAAAAAAC | aphA-3 |
| MKM129 | TGATCCCCAGTAAGTCAAAAAATAG | aphA-3 |
| MKM130 | CATCTGCAGCGATTTTCGTTCGTGAATAC | aad(9) |
| MKM131 | CATCTCGAGAAGGTGTTTCCACCATTTTTTC | aad(9) |
| SPECRup | CGATTTTCGTTCGTGAATAC | aad(9) |
| SPECRdown | AAGGTGTTTCCACCATTTTTTC | aad(9) |
| M13 forward | GTAAAACGACGGCCAG | MCS, pCR2.1TOPO |
| M13 reverse | CAGGAAACAGCTATGAC | MCS, pCR2.1TOPO |
| RT-PCR | ||
| MKM21 | GCGTCCTTTCTATAGAATCGTA | 16S rRNA |
| MKM22 | GATTAAGCTTGCTACTCTATCAGA | 16S rRNA |
| MKM19 | GGTCGCTATATTGGTGAGAACTAT | FTT0232C |
| MKM20 | GGAGCATACCACATAGAGACTTT | FTT0232C |
| Real-time RT-PCR | ||
| MKM26 | GAAGAGAGATTAAAGCTTGATGTTGCT | 16S rRNA |
| MKM27 | GCTTGCTACTCTATCAGAAGGTTGTG | 16S rRNA |
| MKM36p | AAGCTGAAGCATGGTTAGCAAAAGGT | 16S rRNA |
| MKM28 | CAAAATGCTAATGCGTCTTGCA | FTT0232C |
| MKM29 | ATTGGTAATTGCTATTTGTTGACGTT | FTT0232C |
| MKM37p | AGGCATTGGTTTTTTAGCAAAGCCTCTACTCAAA | FTT0232C |
Restriction endonuclease sites are underlined. p, 5′-56-6-carboxyfluorescein, 3′-36-TAMTph-labeled TaqMan probe; MCS, multicloning site.
Cloning FTT0232c and FTT0231c.
In order to express the late acyltransferase ortholog genes in E. coli, F. tularensis FTT0232 and FTT0231c first were cloned into pCR2.1TOPO (Invitrogen) and then were cloned into the expression plasmid pBluescript II SK(−) (Stratagene, La Jolla, CA) as follows. FTT0231c and FTT0232c were amplified from F. tularensis genomic DNA using oligonucleotides MKM5 and MKM8 (FTT0231c) and MKM1 and MKM4 (FTT0232c), producing 1,258- and 1,356-bp amplicons, respectively. Amplicons were ligated into the cloning site of pCR2.1TOPO to get appropriate restriction enzyme sites flanking the DNA fragments. The resulting plasmids were designated pMKM.1 (FTT0232c) and pMKM.2 (FTT0231c). The 1,372-bp EcoRI fragment from pMKM.1 (containing FTT0232c) was ligated into the EcoRI site of pBluescript II SK(−) downstream of the T3 promoter and designated pMKM.11. The 1,301-bp EcoRI fragment from pMKM.2 was ligated similarly into pBluescript II SK(−), and the resulting plasmid was designated pMKM.21. By sequence analysis, both fragments were ligated into pBluescript II SK(−) in the same orientation.
Analysis of high-temperature growth.
Bacteria were grown at 30, 37, or 42°C with agitation at 225 rpm in LB broth in neflometer flasks. Absorbance at 600 nm was recorded using a Spectronic 20 GynSys instrument.
Creation of an E. coli/F. tularensis shuttle and suicide plasmid.
To make targeted mutations by homologous recombination in the F. tularensis genome, a spectinomycin-resistant plasmid was created that replicates in E. coli and conditionally replicates in F. tularensis. This plasmid also contains sacB, the levansucrase gene (which confers sensitivity to sucrose), for counterselection against insertion of the entire plasmid into the genomic DNA; expression of sacB is driven by the groE operon promoter from LVS. Construction of this plasmid is described below.
The spectinomycin adenyltransferase gene, aad(9), encoding resistance to spectinomycin, was PCR amplified from pSPECR (57) as a 1,106-bp fragment with oligonucleotides SPECRup and SPECRdown, omitting the 3′ translational stop codon to allow read-through to downstream open reading frames. The amplicon was subcloned into pCR2.1TOPO, creating pCRspec2. aad(9) was amplified as a 1,106-bp fragment from pCRspec2 using oligonucleotides MKM130 (PstI site at the 5′ end) and MKM131 (XhoI site at the 5′ end) and digested with PstI and XhoI. A 2,650-bp fragment containing sacB and the E. coli origin of replication (oriE) was PCR amplified from pFNLTP10grosacB (a kind gift from Thomas Zhart), a derivative of pFNLTP9 (32) containing sacB and the E. coli origin of replication, using oligonucleotides MKM104b (PstI site at the 5′ end) and MKM105 (XhoI site at the 5′ end). The sacB-oriE amplicon was digested with PstI and XhoI and ligated to the PstI-XhoI-restricted aad(9) amplicon. The ligation reaction was transformed into DH5α competent E. coli cells, and spectinomycin-resistant colonies were selected on LB agar medium. The resultant plasmid, pMKM.217, was isolated and confirmed by digest and sequence analysis.
A 3,730-bp fragment containing the Francisella temperature-sensitive origin of replication and the F. tularensis LVS groE operon promoter was PCR amplified from pFNLTP10grosacB using oligonucleotides MKM106 and MKM107 (both contain an XhoI site at the 5′ end). The amplicon was run on a 0.8% low-melting-point agarose gel, and the desired band was excised and extracted from the agarose. The fragment was digested with XhoI and ligated to pMKM.217 DNA that had been digested with XhoI and treated with Antarctic phosphatase (New England Biolabs). For selection of recombinants containing the Francisella origin of replication, the ligation reaction was transformed into F. tularensis LVS by cryotransformation. Spectinomycin-resistant transformants were selected on CHAB containing spectinomycin at 32°C. Plasmids were isolated from individual colonies using the Qiaprep Spin mini prep kit (QIAGEN). The resultant plasmid, pMKM.219c, was confirmed by digest and sequence analysis.
Cloning an FTT0232c deletion-insertion derivative.
To inactivate FTT0232c, the 5′ and 3′ regions of FTT0232c and flanking DNA sequences, excluding the nucleotides encoding the active-site domains, were amplified, and a gene encoding resistance to kanamycin was inserted between the upstream and downstream DNA sequences as follows. The 5′ region of FTT0232c and flanking DNA sequence was amplified as a 986-bp fragment from F. tularensis genomic DNA using oligonucleotides MKM83 (SmaI at the 5′ end) and MKM86 (BamHI at the 5′ end). The 3′ region of FTT0232c and flanking sequence was amplified from genomic DNA as a 964-bp fragment using oligonucleotides MKM85 (BamHI at the 5′ end) and MKM82 (SmaI at the 5′ end). These two amplicons were ligated using T4 ligase, and the ligation reaction was used as a template for a PCR using oligonucleotides MKM85f and MKM86r. The 1,950-bp amplicon was ligated into pCR2.1TOPO (Invitrogen), and the resultant plasmid, pMKM.16, was confirmed by digest and sequence analysis to contain the upstream and downstream regions of FTT0232c in the correct orientation. The 850-bp SmaI fragment from pUC18K (33), containing the aphA-3 cassette followed by a ribosome binding site and translational start site, was cloned into the SmaI site between the upstream and downstream regions of FTT0232c in order to create a nonpolar insertion in the genomic DNA. Digest and sequence analysis of the resultant plasmid, pMKM.17, confirmed that aphA-3 was inserted in the same orientation as the deletion derivative of FTT0232c.
Using oligonucleotides M13 forward and M13 reverse, which anneal to the multicloning site of pCR2.1TOPO, the interrupted FTT0232c derivative was amplified from pMKM.17 as a 3,000-bp fragment. This amplicon was ligated into the SalI site of pMKM.219c, and the ligation reaction was transformed into E. coli DH5α cells. Spectinomycin- and kanamycin-resistant transformants were selected on LB agar, and plasmid DNA was isolated. The resultant plasmid, pMKM.223, was confirmed by sequence analysis to contain FTT0232c and aphA-3.
Cryotransformation.
F. tularensis bacteria were grown on CHAB or chocolate agar, respectively, for 48 h, collected, and suspended in 0.2 M KCl. Bacteria were centrifuged and washed with 0.2 M KCl two times. After the second wash, bacteria were suspended in 0.2 M KCl at approximately 4 × 1010 CFU ml−1. A volume of 25 μl of cell suspension (approximately 1 × 109 CFU) was added to 25 μl transformation buffer (0.2 M MgSO4, 0.1 M Tris acetate buffer, pH 7.5), and 100 ng DNA was added to the cell suspension. The mixture was incubated at room temperature for 10 min, incubated in liquid nitrogen for 5 min, and finally incubated at room temperature for 10 min to allow cells to thaw. Cells were plated on the appropriate agar medium without selection and were incubated at 32°C overnight. Cells were collected into phosphate-buffered saline (PBS) and spread onto the appropriate medium (chocolate or CHAB) containing appropriate antibiotics and were incubated at 32°C for 24 to 96 h until colonies appeared.
Creating an F. tularensis FTT0232c mutant.
To investigate the function of the FTT0232c-encoded product in F. tularensis lipid A biosynthesis, the FTT0232c gene was inactivated by homologous recombination between genomic DNA and pMKM.223. pMKM.223 DNA was cryotransformed into F. tularensis bacteria, and kanamycin-resistant transformants were selected at the permissive temperature (32°C). The efficiency of transformation was 8.3 × 107 CFU μg of DNA−1, with a range of 4.8 × 107 to 1.3 × 108 CFU μg of DNA−1 (data not shown), and all selected transformants contained pMKM.223, as confirmed by PCR analysis for the presence of the aphA-3 gene (data not shown). To allow homologous recombination to occur between recombinant FTT0232c and genomic DNA, transformants were inoculated into broth medium and were incubated at the permissive temperature with shaking until the culture reached an absorbance at 600 nm of approximately 0.3. By virtue of the temperature-sensitive origin of replication, freely replicating plasmids were cured from the kanamycin-resistant bacterial population when bacterial cultures (approximately 7.5 × 108 CFU) were incubated at 42°C. PCR amplification using oligonucleotides flanking the aphA-3 gene, which anneal within FTT0232c and surrounding sequence, showed that 8 of 13 (61.5%) transformants harbored both a wild-type and a mutated copy of FTT0232c, and 5 of 13 (38.5%) transformants had undergone homologous recombination and contained only a single, mutated copy of the gene. The transformants that had undergone homologous recombination were inoculated onto medium containing 5% sucrose and were incubated at the permissive temperature. One sucrose- and kanamycin-resistant transformant was chosen for further study and was named MKM87. Southern blot analysis confirmed that FTT0232c was interrupted in strain MKM87 and that no parental plasmid DNA remained within the genomic DNA (data not shown).
LPS isolation.
Bacteria were grown as a lawn for approximately 16 h on 10 LB or chocolate agar medium plates with appropriate antibiotics at various temperatures (B178, MKM10, and MKM11 at 37°C and MLK217 at 30°C) and were collected by flooding the plates with PBS and scraping colonies from the surfaces. Cells were centrifuged and suspended in a solution of 6 mM Tris base (Research Products International Corporation, Mt. Prospect, IL), 10 mM EDTA (Fisher Scientific, Fair Lawn, NJ), and 2.0% (wt/vol) sodium dodecyl sulfate (SDS) (Research Products International), pH 6.8, containing 50 μg/ml proteinase K, were incubated at 65°C for 1 h, and then were incubated overnight at 37°C. To remove SDS, samples were precipitated with 0.3 M sodium acetate and 3 volumes cold 100% ethanol, flash cooled in a dry ice-ethanol bath, and incubated overnight at −20°C. Samples were centrifuged for 10 min at 12,000 × g at 4°C, and pellets were suspended in deionized water and precipitated a total of three times. Samples were suspended in water and treated with 80 U micrococcal nuclease (Sigma, St. Louis, MO) for 2 h at 37°C. LPS samples and phenol were equilibrated to 65°C, and an equal volume of phenol was added to the lysates. Samples were mixed, incubated at 65°C for 30 min, cooled on ice, and centrifuged at 3,000 rpm for 10 min at 4°C. The aqueous layer was collected, and the organic layer was back extracted with an equal volume of water; aqueous layers were combined. Samples were precipitated with ethanol three times as described above, and after the last precipitation pellets were suspended in high-performance liquid chromatography-grade water (Fisher Scientific) and lyophilized overnight in a VirTis (Gardiner, NY) BenchTop 2K lyophilizer.
SDS-polyacrylamide gel electrophoresis analysis.
LPS samples were separated on a precast 4 to 20% Tris-HCl polyacrylamide gel (Bio-Rad, Hercules, CA) with SDS and visualized by silver staining as previously described (52).
Acid hydrolysis of LPS-lipid A preparation.
LPS from wild-type E. coli (MLK2), lpxL mutant E. coli (MLK217), the FTT0231c-complemented E. coli lpxL mutant (MKM11), and the FTT0232c-complemented E. coli lpxL mutant (MKM10) (2.6, 2.2, 2.0, and 2.6 mg, respectively) were hydrolyzed in 200 μl 1% acetic acid at 100°C for 2 h. In addition, LPS samples from wild-type and lpxL mutant F. tularensis bacteria (1.9 and 1 mg, respectively) were hydrolyzed under identical conditions. Samples were centrifuged at 4°C for 40 min, and the pellets containing lipid A were washed two times with water and dried under a stream of nitrogen. For further purification, the lipid A pellets were partitioned in 525 μl (10:5:6, vol/vol/vol) CHCl3-CH3OH-H2O, and the bottom organic layers and interfaces were saved and evaporated to dryness under a stream of nitrogen. Alternatively, the E. coli LPS samples (2.5 mg MLK2, 1.8 mg MLK217, and 2.1 mg MKM10) were hydrolyzed under milder conditions using the method of Caroff et al. with some minor modifications (10). LPS samples were treated with 200 μl 10 mM sodium acetate containing 1% SDS at pH 4.5, sonicated for 30 s, and then heated at 100°C for 1 h. Samples then were frozen and lyophilized in a Speed-Vac concentrator. The dry samples were treated with 200 μl of acidified ethanol (a 1:200 [vol/vol] solution of 4 M HCl in ethanol) and centrifuged at 12,000 × g for 15 min at 4°C. Supernatants were removed, and pellets were washed three times with 200 μl ethanol. Lipid A samples then were extracted with 580 μl of a two-phase Bligh/Dyer mixture consisting of CHCl3-CH3OH-H2O (2/2/1.8 by volume) (7). Bottom organic layers and interfaces were saved and evaporated to dryness under a stream of nitrogen.
MSn.
Data for multiple-stage mass spectrometry (MSn) were acquired on an LTQ linear ion trap (LIT) fitted to a vacuum matrix-assisted laser desorption ionization (vMALDI) ion source (Thermo Fisher Scientific, San Jose, CA). The intermediate vMALDI (170-mTorr) ion source uses a 337.7-nm nitrogen laser with a frequency of 20 Hz and energy of 250 μJ per pulse. Lipid A samples were dissolved in 3:1 (vol/vol) CHCl3-CH3OH, and l μl of analyte was mixed with l μl saturated CMBT (6-chloro-3-mercaptobenzothiazole) matrix solution in 3:1 (vol/vol) CHCl3-CH3OH and then spotted on 96- or 384-position stainless steel target plates. Data were acquired in negative- and positive-ion modes using XCalibur 1.4 software scanning a mass range from m/z 700 to 4,000 (in MS mode). To record multiple-stage mass spectra (n = 2 to 4), the following parameters were used: precursor ion isolation width of 3 m/z, normalized collision energy of 40% (the radio frequency amplitude, as a percentage, used to fragment ions), activation Q of 0.25, and activation time of 30 ms. Spectra were recorded using automatic gain control to control the number of laser shots and the automatic spectrum filter tool.
MALDI-TOF MS.
Lipid A was additionally analyzed by MALDI-time-of-flight MS (MALDI-TOF MS) using an Applied Biosystems (Framingham, MA) Voyager DESTR plus instrument. Mass spectra were run in negative-ion, reflectron mode. The instrument was equipped with a nitrogen laser (337 nm) and was operated under delayed extraction conditions: the delay time was 175 ns, and the grid voltage was 64.5% of the full acceleration voltage (20 kV) (21). Lipid A samples were prepared and loaded on a stainless steel target as described above for vMALDI-LIT MS analysis. Typically, 50 to 100 laser shots were used to record each spectrum. Masses were calibrated with an external calibrant consisting of an equimolar mixture of angiotensin I, adrenocorticotropin hormone 1-17 (ACTH 1-17), ACTH 18-39, and ACTH 7-38 (Sigma) to achieve a mass accuracy of <50 ppm in reflectron mode.
MDM isolation.
Heparinized blood was drawn from healthy volunteers according to a protocol approved by the Institutional Review Board at the University of Iowa (8). Peripheral blood mononuclear cells were isolated after dextran sedimentation and Ficoll-Hypaque density gradient separation. Cells were centrifuged at 800 × g, resuspended in RPMI 1640 (Gibco, Carlsbad, CA), and enumerated using a hemacytometer. Viability was assessed by trypan blue exclusion. Peripheral blood mononuclear cells were seeded at 2 × 106 cells/ml in Teflon-coated jars (Savillex, Minnetonka, MN) with 20% autologous serum and were incubated for 7 days at 37°C with 5% CO2; 5% fresh autologous serum was added on day 5. After differentiation, cells were centrifuged and enumerated. MDMs were isolated by adherence of seeding cells onto tissue-culture-treated wells or flasks (Corning, Acton, MA) treated with 1× type IV human placental collagen (Sigma) in RPMI 1640 containing 10% autologous serum; cells were incubated for 24 h at 37°C with 5% CO2. To determine the number of adherent cells (MDMs), one monolayer from each experiment was disrupted with trypsin-EDTA (Gibco) and was incubated at 37°C with 5% CO2 until cells lifted off the surface. Cells were enumerated using a hemacytometer.
MDM infection.
MDM monolayers were infected 10:1 with broth-cultured F. tularensis bacteria for 2 h at 37°C with 5% CO2 in RPMI 1640. Monolayers were washed three times with Hank's balanced salt solution to remove extracellular bacteria, and fresh RPMI 1640 was added. Infected cells were incubated for 4 to 10 h. At each time point, monolayers were disrupted with 2% saponin, and lysates were plated on chocolate agar to determine the multiplicity of infection (MOI). Data are from three separate infections performed in triplicate. F. tularensis growth in RPMI 1640 medium was measured by plating lysates on chocolate agar medium and counting the number of CFU; no bacterial replication in RPMI 1640 was measured (data not shown).
RNA isolation.
RNA was isolated from broth-grown F. tularensis bacteria during mid-log phase (absorbance at 600 nm, ∼0.4) and from intracellular bacteria after 4 and 10 h of MDM infection. TRIzol reagent (Invitrogen) was added to cell pellets or to cell monolayers and was incubated at room temperature for 5 min. An equal volume of chloroform was added to the cell lysates, incubated for 5 min at room temperature, and centrifuged for 15 min. RNA was precipitated from the aqueous phase with 0.3 M ammonium acetate, isopropanol, and 1.0 μl GenElute linear polyacrylamide (Sigma). RNA samples were treated with RQ1 RNase-free DNase (Promega, Madison, WI) to remove contaminating DNA. Reverse transcriptase PCR (RT-PCR) experiments were performed without RT to confirm that DNA was not present in the RNA samples.
RT-PCR.
RT-PCR experiments were performed using the Titan One-Tube RT-PCR system (Roche, Mannheim, Germany). The RNA concentration was determined spectrophotometrically and by gel electrophoresis. Equal amounts of total RNA were used in all RT-PCR experiments. Primers are listed in Table 2.
Real-time RT-PCR.
Real-time RT-PCR experiments were performed using the TaqMan One-Step RT-PCR kit (Roche) and were run on an ABI Prism 7700 sequence detection machine (Applied Biosystems, Foster City, CA). Primers and 6-carboxyfluorescein-, 6-carboxytetramethylrhodamine-labeled probes were designed using Primer Express software (Applied Biosystems) and are listed in Table 2. Reactions were performed in triplicate from two separate RNA isolations, and standard curves were derived for the 16S rRNA and FTT0232c genes from broth-grown bacterial RNA in order to estimate transcript concentration. The slopes of the standard curves for both genes were approximately −3.33, and the R2 values were approximately 0.995. TaqMan reactions were performed without RT in order to analyze DNA contamination in the RNA samples. Data analysis was performed using Microsoft Excel, and the Student t test was used to calculate the significance of differential gene expression.
RESULTS
Search for a late acyltransferase.
The F. tularensis subsp. tularensis SCHU S4 genome database (accession no. AJ749949) was searched using the amino acid sequence of the E. coli laurate transferase, LpxL (accession no. CAA43317). An open reading frame encoding an ortholog of the Kdo-dependent late acyltransferase was identified at nucleotides 246328 to 247227 (FTT0232c). The predicted amino acid sequence encoded by FTT0232c shares 35% identity to E. coli LpxL and 24% identity to E. coli LpxM (accession no. BAB35988). In addition, FTT0232c is 35% identical to the cold-shock-inducible palmitoleoyl transferase of Salmonella enterica serovar Typhimurium (accession no. AE008808.1). FTT0232c is located adjacent to FTT0231c, another open reading frame with significant homology to E. coli LpxL (31%) and LpxM (25%); the predicted amino acid sequences encoded by FTT0232c and FTT0231c are 52% identical to each other. Two regions of conserved amino acid sequence define the active site of late acyltransferase enzymes (37), and homologous regions were found in both of the FTT0232c and FTT0231c predicted amino acid sequences. At the first position, FTT0232c had identical amino acids at 9 of 14 positions (64.3%), and 13 of 18 amino acids (72.2%) were identical at the second position. FTT0231c had 50% (7/14) and 66.7% (12/18) identity to the consensus regions at positions 1 and 2, respectively.
Complementation of the temperature-sensitive growth phenotype.
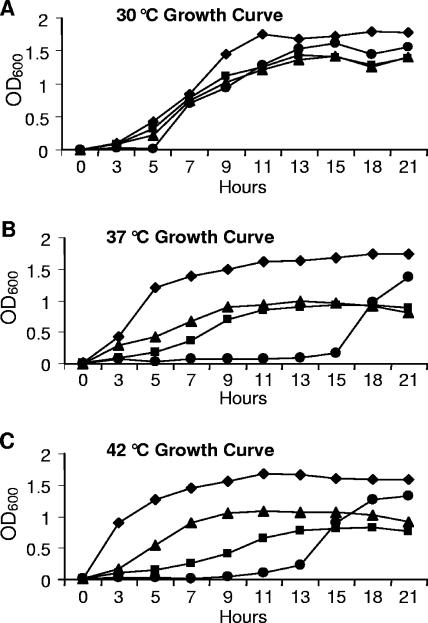
To determine if FTT0231c or FTT0232c encoded late acyltransferase enzymes, complementation of the high-temperature growth defect phenotype of the E. coli lpxL mutant was studied. LpxL is essential for cell viability at temperatures above 33°C, and when exposed to nonpermissive temperatures, cells cease dividing and lose viability within 2 h (26). B178 (wild-type E. coli), MLK217 (E. coli lpxL mutant), MKM11 (MLK217 expressing FTT0231c), and MKM10 (MLK217 expressing FTT0232c) were grown at 30, 37, and 42°C in the presence of appropriate antibiotics, and absorbance at 600 nm was recorded at regular intervals (Fig. 1). All strains grew like wild-type bacteria at 30°C. At 37°C, MLK217 did not begin to grow until 13 to 15 h, as expected. In high concentration, LpxM can functionally substitute for a lack of LpxL by adding a C-14 fatty acid to (Kdo)2-lipid IVA; accumulation of LpxM in the cell is responsible for growth of the lpxL mutant beginning at 13 h (27). In contrast, MKM10 and MKM11 were able to grow at 37°C, although at a sub-wild-type level. At 42°C, lpxL mutant bacteria had an extended 13-h lag phase, while the FTT0232c-complemented mutant strain was able to rescue this phenotype with an approximately 3-h lag phase and a similar rate of growth. The mutant growth defect observed at 42°C was not altered by expression of FTT0231c, as the growth rate was much lower than that of the wild-type or the FTT0232c-complemented strains. MLK217 harboring pBluescript II SK(−) as a control for the vector exhibited the same phenotype as the mutant (data not shown).
FIG. 1.
Complementation of the temperature-sensitive growth phenotype of E. coli lpxL. Cultures were grown at 30°C (A), 37°C (B), and 42°C (C) with agitation at 225 rpm. ⧫, strain B178, wild-type E. coli; •, strain MLK217, E. coli lpxL mutant; ▴, strain MKM10, E. coli lpxL mutant expressing F. tularensis FTT0232c; ▪, strain MKM11, E. coli lpxL mutant expressing F. tularensis FTT0231c. Absorbance was measured by the optical density at 600 nm (OD600).
Mass spectrometric analysis of lipid A isolated from complemented E. coli mutants.
In E. coli, LpxL acylates (Kdo)2-lipid IVA with a lauric acid moiety, and mutation of lpxL results in tetra-acylated lipid A as the major species (14). To determine if F. tularensis FTT0232c encodes a functional late acyltransferase, we compared lipid A isolated from MKM10 (MLK217 expressing F. tularensis FTT0232c) to those isolated from MLK217 (E. coli lpxL mutant) and E. coli wild-type strain B178. All three lipid A species were prepared by isolation of the LPS using a phenol extraction protocol followed by acid hydrolysis of the LPS, yielding polysaccharide and lipid A. The standard acetic acid hydrolysis method was used, although we also prepared lipid A by a milder hydrolysis method using ammonium acetate (10). No significant differences were observed between these samples and the samples prepared using acetic acid.
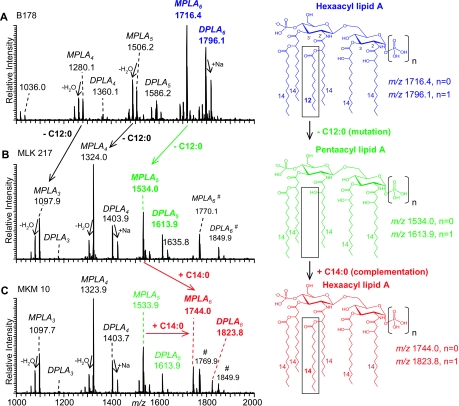
The lipid A fractions isolated by acetic acid hydrolysis first were analyzed by vMALDI-LIT MS in the negative-ion mode, yielding monoisotopic masses (Fig. 2). The mass spectrum obtained from the lipid A species isolated from E. coli wild-type strain B178 (Fig. 2A) was consistent with the lipid A structures previously reported for E. coli K-12 strains (38). As seen in Fig. 2A, two abundant singly charged deprotonated molecular ions, (M-H)−, were observed at m/z 1796.1 and 1716.4 (marked in blue), corresponding to di- and monophosphoryl hexa-acylated lipid A structures (DPLA6 and MPLA6, respectively) containing four 3-hydroxymyristic acids [C14:0(3-OH)], one myristic acid (C14:0), and one lauric acid (C12:0). Other abundant components of the heterogeneous lipid A mixture were observed at m/z 1586.2 and 1506.2, corresponding to penta-acyl DPLA5 and MPLA5, as well as tetra-acyl DPLA4 and MPLA4 at m/z 1360.1 and 1280.1, consisting of one lauric and three or four 3-hydroxymyristic acids, respectively.
FIG. 2.
Negative-ion vMALDI-LIT mass spectra (using an LTQ LIT) of lipid A. vMALDI-LIT MS of lipid A isolated from (A) E. coli wild-type strain B178, (B) E. coli lpxL mutant strain MLK217 lacking a lauric acid in its structure (dodecanoic acid/C12:0 fatty acid; Δ = −182 Da), and (C) MKM10, an E. coli lpxL strain expressing FTT0232c, which complements MLK217 lipid A with a myristic acid (tetradecanoic acid/C14:0 fatty acid; Δ = +210 Da). Complemented lipid A species were observed with (M-H)− at m/z 1744.0 (MPLA) and 1823.8 (DPLA). Lipid A mass peaks are annotated as DPLA and MPLA, respectively, with their number of fatty acid chains and their assigned composition of fatty acids. (B and C) Two hexa-acyl lipid A species (M-H)− at m/z 1849.9 and 1770.1, marked with a pound sign, are shown that are present only in the lpxL mutant and that contain a palmitoleic acid (C16:1) (also see Fig. S1 in the supplemental material and previous related reports by Karow and Georgopoulis and Sunshine et al. [28, 49]). All spectra yielded monoisotopic masses. The numbers 2′, 3′, 2, and 3 refer to the positions of fatty acid substitutions on the distal (2′ and 3′) and proximal (2 and 3) glucosamine rings of the lipid A core shown in the structure (top right).
Figure 2B shows a vMALDI-LIT MS spectrum of lipid A obtained from E. coli lpxL mutant strain MLK217 lacking lauric acid (27). (M-H)− of the major lipid A components of MLK217 appeared 182 Da lower in mass at m/z 1613.9 and 1534.0 (penta-acyl lipid A), m/z 1403.9 and 1324.0 (tetra-acyl lipid A), and m/z 1177.9 and 1097.9 (triacyl lipid A). These molecular ions correspond to DPLA and MPLA structures with fatty acid substituents as follows: five 4× C14:0(3-OH), C14:0; four 4× C14:0(3-OH); and three 3× C14:0(3-OH), respectively. The mass difference of 182 Da between species present in the wild-type and mutant strains is consistent with the loss of a single lauric acid. Strain MLK217 also expressed two lipid A species at m/z 1849.9 and 1770.1 that can be considered side products (Fig. 2B). These peaks were classified as hexa-acyl DPLA and MPLA species containing an extra palmitoleic acid [C16:1] (see Fig. S1 in the supplemental material), consistent with previous fatty acid analysis data reported for the E. coli lpxL mutant (27) and the specificity of LpxP acyltransferase, an LpxL paralog that has been shown to transfer palmitoleic acid to the 2′ secondary acyl chain position of E. coli lipid A (54). Similar observations of lipid A structures bearing additional longer fatty acid chains also have been reported for an S. enterica serovar Typhimurium lpxL mutant (49). Figure 2C shows a negative-ion vMALDI-LIT mass spectrum of the lipid A fraction isolated from complemented strain MKM10 (MLK217 expressing FTT0232c). Several deprotonated molecular ions were observed that were previously detected in the lpxL mutant MLK217. However, in the complemented strain MKM10, two novel (M-H)− peaks emerged at m/z 1823.8 and 1744.0 (marked in red) that were classified as DPLA6 and MPLA6 structures, respectively, consisting of four 3-hydroxymyristic acids and two myristic acids. The latter two novel lipid A peaks showed significant abundance, estimated at about one-third of the base peak intensity. Apparently, complementation of the lpxL mutant with F. tularensis FTT0232c resulted in the addition of an extra fatty acid to the penta-acyl lipid A species at m/z 1613.9 and 1534.0. The mass increase of 210 Da to form the novel hexa-acyl lipid A species at m/z 1823.8 and 1744.0 observed in the complemented strain MKM10 corresponds to the addition of one myristic acid. To obtain even higher resolved peaks for the accurate determination of monoisotopic masses, MALDI-TOF mass spectra were acquired in the negative-ion reflectron mode (see Fig. S2 in the supplemental material). The C-12 monoisotopic masses detected by MALDI-TOF MS and the corresponding assigned structures for E. coli lipid A species are listed in Table 3.
TABLE 3.
Mr observed by MALDI-TOF MS for lipid A structures of E. coli lpxL mutant strain MLK 217 and MKM10 (MLK217 complemented with FTT0232c)
| Mr observeda (exact) | Mr calculated (exact) | ΔMd (ppm) | Lipid structure (fatty acid composition) |
|---|---|---|---|
| 1,825.17b | 1,825.24 | 39 | 2× C14:0, 4× C14:0(3-OH), 2× P |
| 1,745.21 | 1,745.27 | 38 | 2× C14:0, 4× C14:0(3-OH), P |
| 1,614.98 | 1,615.04 | 38 | C14:0, 4× C14:0(3-OH), 2× P |
| 1,535.01 | 1,535.08 | 45 | C14:0, 4× C14:0(3-OH), P |
| 1,404.79 | 1,404.84 | 41 | 4× C14:0(3-OH), 2× P |
| 1,324.82 | 1,324.88 | 43 | 4× C14:0(3-OH), P |
| 1,178.59 | 1,178.65 | 48 | 3× C14:0(3-OH), 2× P |
| 1,098.63 | 1,098.68 | 47 | 3× C14:0(3-OH), P |
| 1,851.23c | 1,851.26 | 11 | C16:1, C14:0, 4× C14:0(3-OH), 2× P |
| 1,771.24c | 1,771.36 | 29 | C16:1, C14:0, 4× C14:0(3-OH), P |
Species were detected in negative-ion mode as (M-H)− by MALDI-TOF MS analysis (monoisotopic masses are reported).
Masses in boldface were detected exclusively in the complemented strain MKM10 (MLK217 complemented with FTT0232c).
Lipid A species that appeared in lpxL mutant strain MLK217 containing additional C16:1 fatty acids.
ΔM, mass difference between the observed and calculated mass of various lipid A species.
The above-described mass spectrometric analysis supports the successful complementation of the E. coli lpxL mutant with FTT0232c and its function as a fatty acid transferase (when expressed in E. coli, FTT0232c encodes a transferase that functions as a myristyl transferase). Further evidence was provided by detailed MSn investigations of the novel complemented DPLA6 and MPLA6 species. Previously, mass spectrometric analyses of lipid A species have been published that used electrospray ionization in combination with multiple-stage fragmentation (6, 16, 30, 36). The mass spectrometric method (vMALDI-LIT-MSn) used in this study for structural assignments of lipid A structures is based on our recently published approach using a vMALDI-LIT instrument (47). The vMALDI-LIT MSn fragmentation patterns of the novel complemented MPLA species observed in strain MKM10 (lpxL mutant MLK217 expressing F. tularensis FTT0232c) confirmed a fatty acid composition of 4× C14:0(3-OH), 2× C14:0 corresponding to the addition of one myristic acid (+210 Da) to the 3-hydroxy myristic acid at the 2′ position of the distal glucosamine, as suggested above. Figure 3A shows a vMALDI-LIT MS2 of the complemented MPLA with the precursor ion selected at m/z 1744.0, yielding fragment ions that represent single- and multiple-bond cleavages. Acyl cleavage patterns are annotated with letters a to f within the spectra, indicating specific positions of acyl chain losses or cross-ring cleavages (see the inset for fragment ion structures). Fragment ions at m/z 1515.6 and 1499.6 arose from the loss of myristic acid (−228 Da; a or b) and 3-hydroxy myristic acid (−244 Da; c) from the precursor ion, respectively. The abundant fragment ion at m/z 1271.5 was assigned to a two-bond cleavage involving the loss of both of these fatty acids. Further loss of a Δ2-C14:1 acyl group (−226 Da; d) from a fragment ion at m/z 1271.5 led to an ion at m/z 1045.5. The Δ2-C14:1 acyl group initially appeared as a 3-hydroxymyristic acid at the 3′ position that was 3-O linked to a myristic acid that was eliminated in an earlier fragmentation process. The MS2 spectrum also revealed a peak at m/z 817.4 that arises from a loss of four acyl groups, i.e., 2× C14:0, C14:0(3-OH) and Δ2-C14:1 (a, b, c, and d).
FIG. 3.
Negative-ion vMALDI-LIT MSn spectra from complemented E. coli strain MKM10 (lpxL mutant MLK217 expressing F. tularensis FTT0232c). Tandem (MS2) and MS3 spectra of the complemented MPLA6 species with (M-H)− at m/z 1744.0 allowed for the identification of an additional myristic acid [C14:0; Δ = +210 Da; structure, 4× C14:0(3-OH), 2× C14:0, P]. (A) MS/MS at m/z 1744.0; (B) MS3 at m/z 1515.6; (C) MS3 at m/z 1271.5; and (D) MS3 at m/z 1045.5. Acyl cleavage patterns are annotated with letters a to f within the spectra, indicating specific positions of acyl chain losses or cross-ring cleavages (also see the inset for fragment ion structures). The asterisk in panel B indicates that the lipid A fragment ion corresponding to the MS3 precursor ion at m/z 1515.6 could result either from an initial loss of C14:0 in the 3′ position (a loss) or from a loss of C14:0 in the 2′ position (b loss; data not shown). Fatty acids at positions 2′ and 3′ refer to the distal glucosamine (see Fig. 2).
To confirm the locations of the fatty acids on the diglucosamine backbone, the major MS2 fragment ions observed at m/z 1515.6, 1271.5, and 1045.5 were selected as further precursors for collisional activation and yielded the vMALDI-LIT MS3 spectra shown in Fig. 3B to D. All of the latter fragment ions were consistent with the assigned complemented lipid A structure. Cross-ring cleavages (annotated as cleavage f) are designated 0,4A2-type fragments according to the nomenclature of Costello and Vath (17) and are particularly useful for structural analysis. For example, the MS3 spectrum of precursor ion m/z 1045.5 (Fig. 3D) showed an abundant fragment ion at m/z 718.2 that arises from a 0,4A2-type cross-ring cleavage (f) and that can be generated only if the complemented C14:0 fatty acid was 3-O linked to the C14:0(3-OH) acyloxy group in the 2′ position of the diglucosamine backbone. Moreover, additional multiple-stage (MS4) ion trap spectra were recorded, and these data were consistent with that for the assigned structures (data not shown). For comparison and consistency, the vMALDI-LIT MSn ion trap data of the complemented strain MKM10 were compared to those for fragmentation patterns from previously characterized E. coli wild-type hexa-acyl lipid species (data not shown) as well as those reported by others (6).
We also investigated the DPLA6 species at m/z 1823.8 that emerged in strain MKM10 by acquisition of LIT-MSn spectra (see Fig. S3 in the supplemental material). The resulting MSn fragmentation patterns confirmed an additional second phosphate group for this novel hexaacyl lipid A structure bearing four 3-hydroxymyristic acids and two myristic acids (see Fig. S3 in the supplemental material for a more detailed description). These in-depth MSn investigations of the lipid species at m/z 1744.0 and m/z 1823.9 proved that the complementation experiment expressing the FTT0232c gene in an E. coli lpxL mutant (MKM10) successfully incorporated an additional myristic acid.
The function of the FTT0232c gene product also was investigated by expressing the FTT0232c gene in an E. coli lpxM mutant (MKM13) (27). Lipid A was isolated from an E. coli lpxM mutant, MLK1067, and from MKM13 (MLK1067 expressing FTT0232c) and was analyzed by vMALDI-LIT MS (data not shown). However, strain MKM13 showed the same, unchanged lipid A distribution, as was observed for the lpxM mutant strain MLK1067, indicating that there was no functional activity of FTT0232c within the lpxM mutant strain.
In summary, consistent with the mass spectrometric data described above, expression of FTT0232c in an E. coli lpxL mutant was responsible for acylating lipid A with a myristic acid. FTT0232c encodes an acyltransferase capable of utilizing available myristic acid in lipid A assembly in E. coli; moreover, FTT0232c did not function as an acyltransferase in an E. coli lpxM mutant background.
In addition to the F. tularensis FTT0232c complementation studies, a second gene, FTT0231c, was expressed in the E. coli lpxL mutant (MKM11). The lipid A from MKM11 was analyzed by vMALDI-LIT MS in both the positive- and negative-ion modes (see Fig. S4 in the supplemental material). However, unlike the FTT0232c complemented strain, no new lipid A species were observed, and no follow-up studies were carried out.
Mass spectrometric analysis of lipid A isolated from an F. tularensis FTT0232c mutant.
To better define the activity of FTT0232c in F. tularensis, the lipid A from lpxL mutant bacteria was analyzed by MS in both the negative- and positive-ion modes (Fig. 4). Compared to the mass spectra obtained from the lipid A of the wild-type strain (Fig. 4A and C), the structure of which has been previously reported by us and others (36, 53), the mass spectra obtained from the mutant strain (Fig. 4B and D) showed many similar features and a few major differences. First, and to our initial surprise, the negative-ion MS spectra of the lipid A preparations from both the mutant and wild type appear nearly identical (Fig. 4A and B), and these data are consistent with those for the lipid A structures previously reported (36). Briefly, the major lipid A from the wild-type strain consists of a diglucosamine backbone bearing four fatty acids: three 3-hydroxy stearic acids, C18:0(3-OH) (in 2, 3, and 2′ positions), and one palmitic acid, C16:0, linked to the hydroxy group of the 3-hydroxy stearic acid at the 2′ position. These tetra-acyl lipid A species are further modified with a phosphate and galactosamine, yielding the deprotonated molecular ions (M-H)− at m/z 1504 and 1665. However, upon closer inspection of the mass region corresponding to minor triacyl lipid A species (m/z 1200 to 1300), the mutant strain contains a peak at m/z 1237.8 not seen with the wild-type strain. This peak corresponds to a triacyl lipid A in which one of the remaining 3-hydroxy stearic chains has been replaced with a 3-hydroxy palmitic acid [C16:0(3-OH)]. Second, in the positive-ion mode, mass spectra obtained from these same lipid A preparations (Fig. 4C and D) provided more striking differences between the wild-type and mutant strain. As expected, the wild-type strain contained molecular ions for three distinct tetra-acyl lipid A species that exist in multiple states, primarily monosodiated, at m/z 1528.0, 1448.3, and 1392.2, the latter two of which do not contain phosphate (Fig. 4, see structure inset; also see Table S1 in the supplemental material for details). In contrast, the mass spectrum from the mutant strain lipid A showed both a significantly decreased abundance of these tetra-acyl lipid A species and the presence of a new, highly abundant nonphosphorylated triacyl lipid A species [2× C18:0(3-OH), C16:0(3-OH)] at m/z 1182.0 (Fig. 4D). Interestingly, the tetra-acyl species that was previously observed at m/z 1392.2 [2× C18:0(3-OH), C16:0(3-OH), C14:0] was no longer present in the mutant strain (Fig. 4D). The lack of this tetra-acyl species and the presence of the new major triacyl lipid A in the mutant at m/z 1182.0 in positive-ion mode is consistent with the loss of a myristic acid. The structure of the triacyl lipid A at m/z 1182.0 subsequently was confirmed by MSn analysis (see Fig. S5 in the supplemental material) and clearly defines this novel triacyl lipid A as containing two N-linked 3-hydroxy stearic acids at the 2 and 2′ positions and one O-linked 3-hydroxy palmitic acid at the 3 position. Only a small percentage of this major mutant triacyl species remains phosphorylated, presumably at the 1 position, as would be expected from the lipid X precursor (diacylglucosamine 1-phoshate), and it appears as a small peak at m/z 1237.8 in the negative-ion MALDI-MS spectrum (Fig. 4B). Nevertheless, the positive-ion MS results clearly indicate successful mutation of FTT0232c in F. tularensis and its effect on the lipid A structure, consistent with loss of LpxL acyltransferase activity within the mutant bacteria.
FIG. 4.
Negative- and positive-ion vMALDI-LIT mass spectra of lipid A (F. tularensis). Shown are negative- and positive-ion mass spectra of lipid A isolated from F. tularensis wild-type strain 1547-57 (A and C) and from an F. tularensis FTT0232c mutant strain (B and D). While no significant differences between wild-type and mutant strains were observed in negative-ion mode, the positive-ion mode spectra contain several major differences, including the presence of a major (base) peak for the F. tularensis FTT0232c mutant at m/z 1182.0 that corresponds to a new triacyl lipid A species [composition, 2× C18:0(3-OH)2′,2 and C16:0(3-OH)3]. Chemical structures of key lipid A species are depicted for clarification. For further details of lipid A composition, see Table S1 in the supplemental material.
Intracellular growth of F. tularensis.
Human MDM cells were infected with F. tularensis bacteria at a 10:1 (bacteria:MDM) MOI. Bacteria were allowed to settle by gravity onto the MDMs for 2 h, and extracellular bacteria were washed from the monolayer; this time point was designated t0. At t0, monolayers were disrupted with 2% saponin to determine the actual MOI after 2 h of phagocytosis; at this time, the MOI was approximately 0.33 bacteria per MDM (Fig. 5). Similarly, at t4 and t10 (4 and 10 h, respectively, after t0), cell monolayers were disrupted with 2% saponin, and lysates were inoculated onto medium to determine intracellular survival. At t4, approximately 26% of bacteria ingested at t0 were present intracellularly. This population of bacteria represents the number of bacteria that survived initial intracellular killing and bacterial replication within the first 4 h after extracellular bacteria were removed from the monolayers. Between t4 and t10, intracellular bacteria replicated 2.9-fold (intracellular replication rate of 0.48 per h).
FIG. 5.
F. tularensis intracellular survival during infection of MDM. MDM monolayers were infected at an MOI of 10 bacteria to 1 macrophage. After 2 h of phagocytosis, extracellular bacteria were washed from the monolayers. Monolayers were disrupted 0, 4, and 10 h after removal of extracellular bacteria to enumerate intracellular bacteria by counting the number of CFU. Data are from three independent experiments, and standard errors of the means were used to derive error bars.
Expression of FTT0232c.
Expression of F. tularensis FTT0232c during growth in broth medium or during growth within MDMs was examined using semiquantitative RT-PCR. Total RNA (100 ng) was used in each RT-PCR experiment, and the 16S rRNA gene served as an internal control for bacterial RNA concentration, as comparisons of gene expression were normalized to 16S rRNA for each growth condition. Using this method, there were more FTT0232c mRNA transcripts from intracellular F. tularensis bacteria than from bacteria grown in broth medium (data not shown).
Differences in gene expression during in vitro and intracellular growth were quantified using TaqMan real-time RT-PCR. Reactions were performed with high-quality RNA samples, in triplicate, from two separate RNA isolations. After 4 h of infection, there were 2.5-fold ± 0.2-fold more FTT0232c mRNA transcripts in intracellular F. tularensis bacteria than in broth-grown bacteria (Fig. 6). After 10 h of infection, there were 2.9-fold ± 0.3-fold more FTT0232c transcripts in intracellular F. tularensis bacteria than in broth-grown bacteria. At 4 and 10 h of infection, each fold increase in mRNA is significant (P < 0.005).
FIG. 6.
Ratio of expression of F. tularensis genes during MDM infection to that during in vitro culture. Real-time RT-PCR experiments for each RNA sample were done in triplicate from two RNA samples. RNA samples were collected from infection of human MDM monolayers after 4 (filled bars) and 10 (open bars) h of infection and from in vitro-cultured bacteria growing in mid-logarithmic phase. The ratio of transcripts results from the amount of mRNA from MDM infection, normalized to 16S rRNA concentration, divided by the amount of product from in vitro-cultured F. tularensis bacteria at each time point. The increase in transcript for both genes was significant (P < 0.005) compared to the level of 16S rRNA transcripts at 4 and 10 h of MDM infection.
DISCUSSION
LPS is the major component of the outer membrane of gram-negative bacteria, and LPS/myeloid differentiation protein 2 complexes interact with TLR4, stimulating host cells to release proinflammatory cytokines (9, 22, 39). A number of modifications of lipid A structure affect biological activity, including the length of acyl chains, degree of acylation, absence of terminal phosphates, and modification of either phosphate residue to contain an arabinosamine or galactosamine residue (39, 51). F. tularensis LPS has distinctly different physical features that appear to be biologically relevant. Previously, we reported the structure of lipid A from F. tularensis subsp. holarctica strain 1547-57 to elucidate the basis for biological inactivity of Francisella LPS (36). The lipid A of F. tularensis subsp. holarctica strain 1547-57 is primarily tetra-acylated, although minor lipid A species containing an additional 16 and 18 carbon fatty acids are present (36). Moreover, these fatty acids are asymmetrically distributed on the diglucosamine molecule, and a phosphogalactosamine residue is present at the 1 position of the glucosamine backbone (36). In the context of the unusual structure of F. tularensis lipid A, it is not surprising that the LPS does not engage LPS binding protein and therefore does not interact with TLR4, circumventing the usual mechanism of endotoxin stimulation of the immune system (4, 11). In addition, F. tularensis LPS does not act as an antagonist against immune system activation by other LPS molecules (2, 43). Lack of interaction with TLR4 may present a means of stealth entry into the mammalian host to establish infection.
Studies by Wang et al. (55, 56) used complementation in E. coli with Francisella genes to demonstrate functions of the Francisella novicida lipid A biosynthesis genes lpxE and lpxF. In the current study, we identified a gene encoding a homolog of the E. coli laurate transferase, LpxL, in the F. tularensis genome. Within the predicted FTT0232c amino acid sequence were two consensus sequences common to many fatty acyltransferase proteins, indicating that the protein may function as a fatty acyltransferase (37). Overexpression of FTT0232c partially rescued the temperature-sensitive growth defect of E. coli lpxL, presumably because, as the MS data indicated, a myristic acid rather than a lauric acid was added to the mutant (Kdo)2-lipid IVA structure of MKM10. Addition of a myristoyl rather than a lauroyl fatty acid to (Kdo)2-lipid IVA likely would alter cell membrane fluidity and integrity, thus affecting the ability to grow at increased temperatures. Alternatively, as the relative ion abundances of the fully hexa-acylated species were significantly lower than those of the incomplete acylated states (i.e., triacylated lipid A to penta-acylated lipid A) (Fig. 2C) in the complemented lpxL mutant MKM10 compared to those of the wild-type strain (Fig. 2A), secondary acylation also was compromised, potentially contributing to the incomplete rescued phenotype.
In this study, MALDI-based MS methods were employed to examine changes in structurally related lipid A species obtained from E. coli and F. tularensis wild-type and mutant strains that differ in their fatty acid substitution patterns. While one cannot generally assume that changes in relative ion abundances of molecular species observed by MS correlate with their molar abundances, we have previously shown that MALDI MS of heterogeneous LPS and lipid A mixtures can be used for this purpose under conditions in which one is examining relatively minor structural perturbations that do not involve a change in the charge state (23). Therefore, it is with this assumption (and limitations) that relative changes in molecular ion abundances among closely related lipid A species have been used to make semiquantitative arguments regarding the fatty acylation status of lipid A species among the various E. coli and F. tularensis wild-type and mutant strains.
As explained in the previous section, F. tularensis FTT0232c successfully acylated E. coli lpxL mutant lipid A with a myristic acid. However, the major component of F. tularensis (strain 1547-57) lipid A is comprised of a monophosphoryl diglucosamine backbone bearing three C18:0(3-OH) and one C16:0 fatty acid and a galactosamine moiety. An additional component of F. tularensis strain 1547-57 and LVS (ATCC 29684) lipid A contains two 3-hydroxy stearic, one 3-hydroxy palmitic, and one myristic fatty acid (36, 53). To gain more insight into F. tularensis lipid A biosynthesis, we generated an F. tularensis mutant lacking a functional FTT0232c gene (lpxL mutant). As determined by in-depth MS of the isolated lipid A species, this late acyltransferase mutant expressed a novel triacyl lipid A species containing two 3-hydroxy stearic acids and one 3-hydroxy palmitic acid lacking a single myristic acid at a position consistent with the specificity of an LpxL late acyltransferase. Only minor triacyl lipid A species bearing three 3-hydroxy stearic acids were observed in the F. tularensis FTT0232c mutant. One hypothesis to explain these observations is that in wild-type bacteria, this latter lipid A predominantly gets replaced with a C16:0 fatty acid on the hydroxy group of 3-hydroxy stearic acid attached to the 2′ position. This species yields a tetra-acyl lipid A containing three 3-hydroxy stearic groups and one palmitic acyl group. Possibly, FTT0232c functions only as a myristic acid transferase and not as a palmitic acid transferase in the normal F. tularensis background. Besides that of FTT0232c, a separate LpxL also may exist with different fatty acid specificities.
The second potential acyltransferase gene, FTT0231c, did not appear to rescue the E. coli LpxL mutant when complemented into MLK217 (i.e., strain MKM11) at 42°C, as shown by the lack of a change in the slope of the growth curve at the earlier time points (<6 h) (Fig. 1C). The MS data also showed no difference between the lipid A species obtained from the MLK217 (lpxL mutant) and MKM11 (MLK217 complemented with FTT0231c) strains, as judged by their MALDI-MS profiles (see Fig. S4 in the supplemental material).
Complementation experiments with the E. coli lpxM mutant with FTT0232c were unsuccessful; expression of FTT0232c in the E. coli lpxM mutant (MLK1067) was not sufficient to complement the phenotype of this lipid A mutant (data not shown). Lack of complementation of the lpxM mutation with FTT0232c may result from differences in substrate specificity. F. tularensis mutational analysis and expression experiments are ongoing to determine the exact function of FTT0232c in F. tularensis; however, data presented here indicate that FTT0232c encodes a functional late acyltransferase.
Some gram-negative bacteria alter lipid A structure in response to temperature and the microenvironment. Yersinia pestis modifies lipid A structure according to temperature; at 37°C, the lipid A is mostly tetra-acylated and is unable to stimulate release of TNF-α, whereas at 21°C the majority of lipid A is hexa-acylated and is able to stimulate TNF-α secretion from human monocytes (3, 25, 29, 40). Haemophilus influenzae regulates lipid A acylation in response to the microenvironment by increasing expression of the lpxL gene during infection of human airway epithelial xenografts compared to the level of expression with in vitro growth (50). H. influenzae lpxL elaborates a tetra-acyl lipid A that is unable to colonize human bronchial xenografts or produce otitis media in the chinchilla middle ear infection model (18, 31, 50).
We reasoned that F. tularensis might alter its lipid A as a function of its microenvironment. Expression of the F. tularensis FTT0232c gene was analyzed in broth medium and during growth inside human MDM cells. Semiquantitative RT-PCR and quantitative TaqMan real-time RT-PCR experiments confirmed that expression of FTT0232c was increased by approximately 2.5- and 3-fold (P < 0.005) during MDM infection (4 and 10 h, respectively) compared to expression during in vitro growth. After 24 h of infection with F. tularensis, the human macrophage monolayers were no longer intact, and therefore gene expression was not analyzed at this time point. The fact that FTT0232c expression increased during MDM infection relative to growth in broth medium may indicate that its gene product acylates lipid A during intracellular growth. Species acylated at higher levels were previously reported to be found in F. tularensis lipid A preparations, although they were observed at significantly lower abundances than were the tetra-acylated species (36). The ability of the organism to modulate expression of FTT0232c may allow F. tularensis to regulate acylation of lipid A in response to the microenvironment and potentially mediate a survival advantage for the bacterium.
In conclusion, we identified a late acyltransferase enzyme (LpxL) of F. tularensis. Elucidation of LPS biosynthesis and structure in vitro and in vivo may provide insights into the mechanisms of F. tularensis pathogenesis and the immune response to infection.
Supplementary Material
Acknowledgments
This work was supported by a program project grant from National Institutes of Health grant no. AI44642-05.
We thank Mark P. Simons for monocyte isolation, Deborah Post and Nancy Phillips for helpful discussions, and William Nauseef for review of the manuscript. We also acknowledge Thermo Fisher Scientific for providing a vMALDI-LTQ mass spectrometer for evaluation.
Editor: F. C. Fang
Footnotes
Published ahead of print on 27 August 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Abd, H., T. Johansson, I. Golovliov, G. Sandstrom, and M. Forsman. 2003. Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl. Environ. Microbiol. 69:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancuta, P., T. Pedron, R. Girard, G. Sandstrom, and R. Chaby. 1996. Inability of the Francisella tularensis lipopolysaccharide to mimic or to antagonize the induction of cell activation by endotoxins. Infect. Immun. 64:2041-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aussel, L., H. Therisod, D. Karibian, M. B. Perry, M. Bruneteau, and M. Caroff. 2000. Novel variation of lipid A structures in strains of different Yersinia species. FEBS Lett. 465:87-92. [DOI] [PubMed] [Google Scholar]
- 4.Barker, J. H., J. P. Weiss, M. A. Apicella, and W. M. Nauseef. 2006. Basis for the failure of Francisella tularensis lipopolysaccharide to prime human polymorphonuclear leukocytes. Infect. Immun. 74:3277-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron, G. S., and F. E. Nano. 1998. MglA and MglB are required for the intramacrophage growth of Francisella novicida. Mol. Microbiol. 29:247-259. [DOI] [PubMed] [Google Scholar]
- 6.Bedoux, G., K. Vallee-Rehel, O. Kooistra, U. Zahringer, and D. Haras. 2004. Lipid A components from Pseudomonas aeruginosa PAO1 (serotype O5) and mutant strains investigated by electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 39:505-513. [DOI] [PubMed] [Google Scholar]
- 7.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 8.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 97:77-89. [PubMed] [Google Scholar]
- 9.Caroff, M., and D. Karibian. 2003. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 338:2431-2447. [DOI] [PubMed] [Google Scholar]
- 10.Caroff, M., A. Tacken, and L. Szabo. 1988. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the Bordetella pertussis endotoxin. Carbohydr. Res. 175:273-282. [DOI] [PubMed] [Google Scholar]
- 11.Chen, W., R. Kuo Lee, H. Shen, M. Busa, and J. W. Conlan. 2004. Toll-like receptor 4 (TLR4) does not confer a resistance advantage on mice against low-dose aerosol infection with virulent type A Francisella tularensis. Microb. Pathog. 37:185-191. [DOI] [PubMed] [Google Scholar]
- 12.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2005. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect. Immun. 73:5892-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72:3204-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clementz, T., J. J. Bednarski, and C. R. Raetz. 1996. Function of the htrB high temperature requirement gene of Escherichia coli in the acylation of lipid A: HtrB catalyzed incorporation of laurate. J. Biol. Chem. 271:12095-12102. [DOI] [PubMed] [Google Scholar]
- 15.Clementz, T., Z. Zhou, and C. R. Raetz. 1997. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. Acylation by MsbB follows laurate incorporation by HtrB. J. Biol. Chem. 272:10353-10360. [DOI] [PubMed] [Google Scholar]
- 16.Corsaro, M. M., F. D. Piaz, R. Lanzetta, and M. Parrilli. 2002. Lipid A structure of Pseudoalteromonas haloplanktis TAC 125: use of electrospray ionization tandem mass spectrometry for the determination of fatty acid distribution. J. Mass Spectrom. 37:481-488. [DOI] [PubMed] [Google Scholar]
- 17.Costello, C. E., and J. E. Vath. 1990. Tandem mass spectrometry of glycolipids. Methods Enzymol. 193:738-768. [DOI] [PubMed] [Google Scholar]
- 18.DeMaria, T. F., M. A. Apicella, W. A. Nichols, and E. R. Leake. 1997. Evaluation of the virulence of nontypeable Haemophilus influenzae lipooligosaccharide htrB and rfaD mutants in the chinchilla model of otitis media. Infect. Immun. 65:4431-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 20.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson, B. W., J. J. Engstrom, C. M. John, W. Hines, and A. M. Falick. 1997. Characterization of bacterial lipooligosaccharides by delayed extraction matrix-assisted laser desorption ionization time-of flight mass spectrometry. J. Am. Soc. Mass Spectrom. 8:645-658. [Google Scholar]
- 22.Gioannini, T. L., A. Teghanemt, D. Zhang, N. P. Coussens, W. Dockstader, S. Ramaswamy, and J. P. Weiss. 2004. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc. Natl. Acad. Sci. USA 101:4186-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goon, S., B. Schilling, M. V. Tullius, B. W. Gibson, and C. R. Bertozzi. 2003. Metabolic incorporation of unnatural sialic acids into Haemophilus ducreyi lipooligosaccharides. Proc. Natl. Acad. Sci. USA 100:3089-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray, C. G., S. C. Cowley, K. K. Cheung, and F. E. Nano. 2002. The identification of five genetic loci of Francisella novicida associated with intracellular growth. FEMS Microbiol. Lett. 215:53. [DOI] [PubMed] [Google Scholar]
- 25.Hitchen, P. G., J. L. Prior, P. C. Oyston, M. Panico, B. W. Wren, R. W. Titball, H. R. Morris, and A. Dell. 2002. Structural characterization of lipo-oligosaccharide (LOS) from Yersinia pestis: regulation of LOS structure by the PhoPQ system. Mol. Microbiol. 44:1637-1650. [DOI] [PubMed] [Google Scholar]
- 26.Karow, M., O. Fayet, A. Cegielska, T. Ziegelhoffer, and C. Georgopoulos. 1991. Isolation and characterization of the Escherichia coli htrB gene, whose product is essential for bacterial viability above 33°C in rich media. J. Bacteriol. 173:741-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karow, M., O. Fayet, and C. Georgopoulos. 1992. The lethal phenotype caused by null mutations in the Escherichia coli htrB gene is suppressed by mutations in the accBC operon, encoding two subunits of acetyl coenzyme A carboxylase. J. Bacteriol. 174:7407-7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karow, M., and C. Georgopoulos. 1992. Isolation and characterization of the Escherichia coli msbB gene, a multicopy suppressor of null mutations in the high-temperature requirement gene htrB. J. Bacteriol. 174:702-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawahara, K., H. Tsukano, H. Watanabe, B. Lindner, and M. Matsuura. 2002. Modification of the structure and activity of lipid A in Yersinia pestis lipopolysaccharide by growth temperature. Infect. Immun. 70:4092-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kussak, A., and A. Weintraub. 2002. Quadrupole ion-trap mass spectrometry to locate fatty acids on lipid A from gram-negative bacteria. Anal. Biochem. 307:131-137. [DOI] [PubMed] [Google Scholar]
- 31.Lee, N. G., M. G. Sunshine, J. J. Engstrom, B. W. Gibson, and M. A. Apicella. 1995. Mutation of the htrB locus of Haemophilus influenzae nontypable strain 2019 is associated with modifications of lipid A and phosphorylation of the lipo-oligosaccharide. J. Biol. Chem. 270:27151-27159. [PubMed] [Google Scholar]
- 32.Maier, T. M., A. Havig, M. Casey, F. E. Nano, D. W. Frank, and T. C. Zahrt. 2004. Construction and characterization of a highly efficient Francisella shuttle plasmid. Appl. Environ. Microbiol. 70:7511-7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ménard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohara, Y., T. Sato, and M. Homma. 1996. Epidemiological analysis of tularemia in Japan (yato-byo). FEMS Immunol. Med. Microbiol. 13:185-189. [DOI] [PubMed] [Google Scholar]
- 35.Parker, R. R., E. A. Steinhaus, G. M. Kohls, and W. L. Jellison. 1951. Contamination of natural waters and mud with Pasteurella tularensis and tularemia in beavers and muskrats in the northwestern United States. Bull. Natl. Inst. Health 193:1-161. [PubMed] [Google Scholar]
- 36.Phillips, N. J., B. Schilling, M. K. McLendon, M. A. Apicella, and B. W. Gibson. 2004. Novel modification of lipid A of Francisella tularensis. Infect. Immun. 72:5340-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Post, D. M., N. J. Phillips, J. Q. Shao, D. D. Entz, B. W. Gibson, and M. A. Apicella. 2002. Intracellular survival of Neisseria gonorrhoeae in male urethral epithelial cells: importance of a hexaacyl lipid A. Infect. Immun. 70:909-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raetz, C. R. 1993. Bacterial endotoxins: extraordinary lipids that activate eucaryotic signal transduction. J. Bacteriol. 175:5745-5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raetz, C. R., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebeil, R., R. K. Ernst, B. B. Gowen, S. I. Miller, and B. J. Hinnebusch. 2004. Variation in lipid A structure in the pathogenic Yersiniae. Mol. Microbiol. 52:1363-1373. [DOI] [PubMed] [Google Scholar]
- 41.Rietschel, E. T., T. Kirikae, F. U. Schade, U. Mamat, G. Schmidt, H. Loppnow, A. J. Ulmer, U. Zahringer, U. Seydel, F. Di Padova, et al. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8:217-225. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 43.Sandström, G., A. Sjostedt, T. Johansson, K. Kuoppa, and J. C. Williams. 1992. Immunogenicity and toxicity of lipopolysaccharide from Francisella tularensis LVS. FEMS Microbiol. Immunol. 5:201-210. [DOI] [PubMed] [Google Scholar]
- 44.Sanford, J. P. 1983. Landmark perspective: tularemia. JAMA 250:3225-3226. [DOI] [PubMed] [Google Scholar]
- 45.Santic, M., M. Molmeret, and Y. Abu Kwaik. 2005. Modulation of biogenesis of the Francisella tularensis subsp. novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-gamma. Cell. Microbiol. 7:957-967. [DOI] [PubMed] [Google Scholar]
- 46.Saslaw, S., H. T. Eigelsbach, J. A. Prior, H. E. Wilson, and S. Carhart. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Int. Med. 107:702-714. [DOI] [PubMed] [Google Scholar]
- 47.Schilling, B., M. K. McLendon, N. J. Phillips, M. A. Apicella, and B. W. Gibson. 2007. Characterization of lipid A acylation patterns in Francisella tularensis, Francisella novicida, and Francisella philomiragia using multiple-stage mass spectrometry and matrix-assisted laser desorption/ionization on an intermediate vacuum source linear ion trap. Anal. Chem. 79:1034-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Somerville, J. E., Jr., L. Cassiano, B. Bainbridge, M. D. Cunningham, and R. P. Darveau. 1996. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J. Clin. Investig. 97:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sunshine, M. G., B. W. Gibson, J. J. Engstrom, W. A. Nichols, B. D. Jones, and M. A. Apicella. 1997. Mutation of the htrB gene in a virulent Salmonella typhimurium strain by intergeneric transduction: strain construction and phenotypic characterization. J. Bacteriol. 179:5521-5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swords, W. E., D. L. Chance, L. A. Cohn, J. Shao, M. A. Apicella, and A. L. Smith. 2002. Acylation of the lipooligosaccharide of Haemophilus influenzae and colonization: an htrB mutation diminishes the colonization of human airway epithelial cells. Infect. Immun. 70:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teghanemt, A., D. Zhang, E. N. Levis, J. P. Weiss, and T. L. Gioannini. 2005. Molecular basis of reduced potency of underacylated endotoxins. J. Immunol. 175:4669-4676. [DOI] [PubMed] [Google Scholar]
- 52.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 53.Vinogradov, E., M. B. Perry, and J. W. Conlan. 2002. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 269:6112-6118. [DOI] [PubMed] [Google Scholar]
- 54.Vorachek-Warren, M. K., S. Ramirez, R. J. Cotter, and C. R. Raetz. 2002. A triple mutant of Escherichia coli lacking secondary acyl chains on lipid A. J. Biol. Chem. 277:14194-14205. [DOI] [PubMed] [Google Scholar]
- 55.Wang, X., M. J. Karbarz, S. C. McGrath, R. J. Cotter, and C. R. Raetz. 2004. MsbA transporter-dependent lipid A 1-dephosphorylation on the periplasmic surface of the inner membrane: topography of Francisella novicida LpxE expressed in Escherichia coli. J. Biol. Chem. 279:49470-49478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, X., S. C. McGrath, R. J. Cotter, and C. R. Raetz. 2006. Expression cloning and periplasmic orientation of the Francisella novicida lipid A 4′-phosphatase LpxF. J. Biol. Chem. 281:9321-9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitby, P. W., D. J. Morton, and T. L. Stull. 1998. Construction of antibiotic resistance cassettes with multiple paired restriction sites for insertional mutagenesis of Haemophilus influenzae. FEMS Microbiol. Lett. 158:57-60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.