Abstract
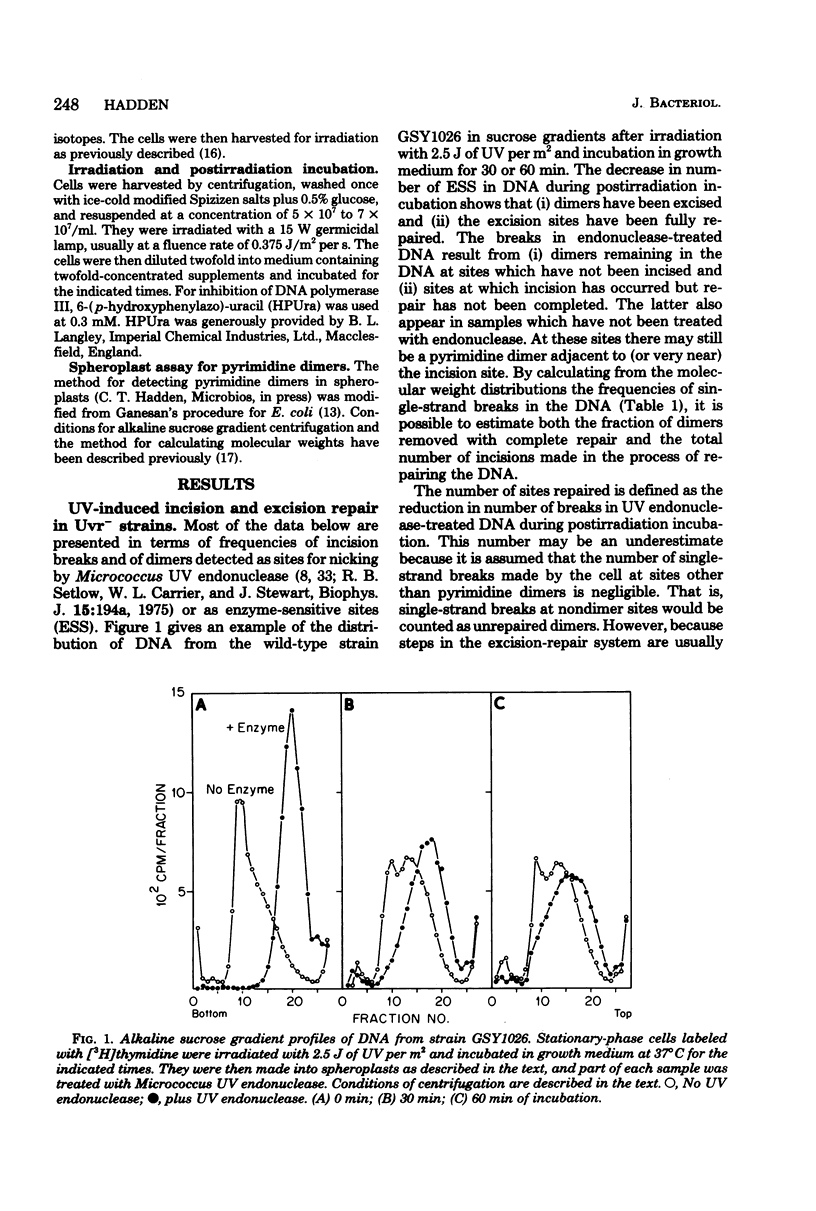
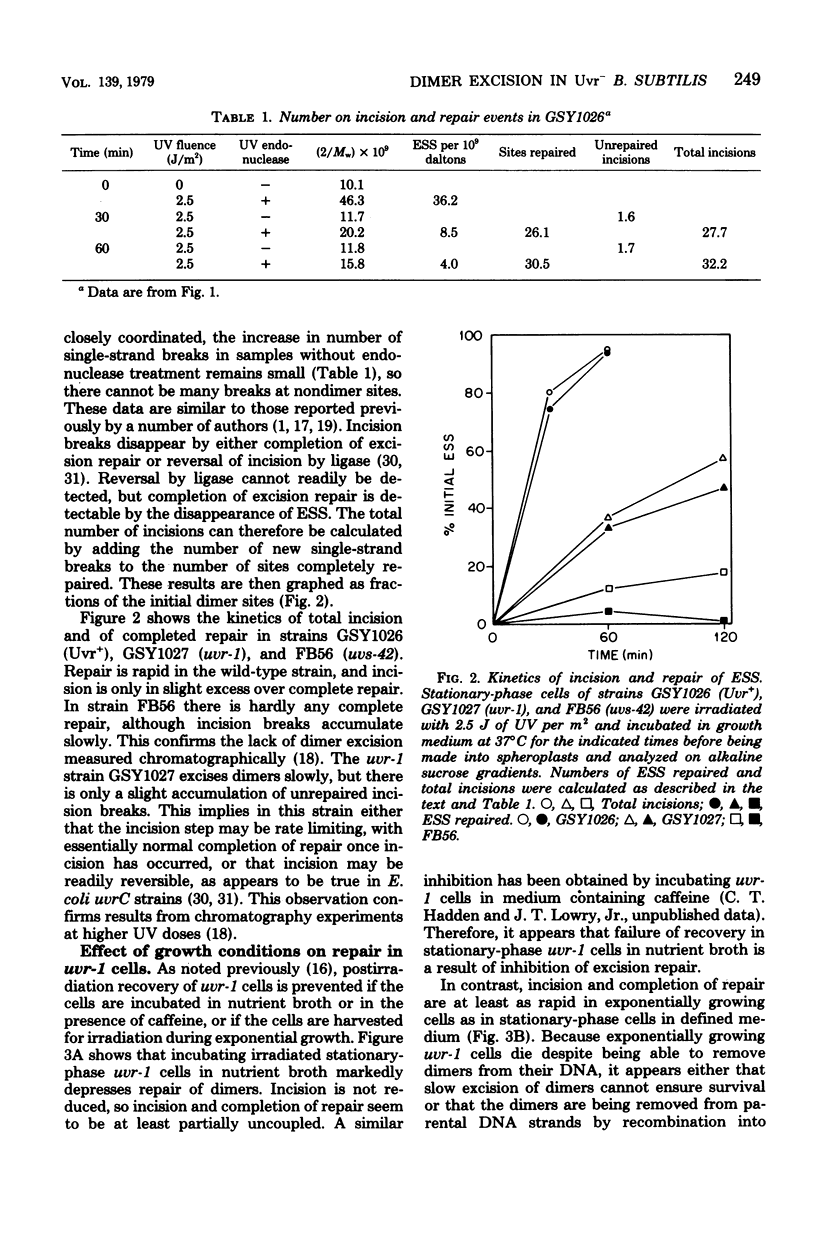
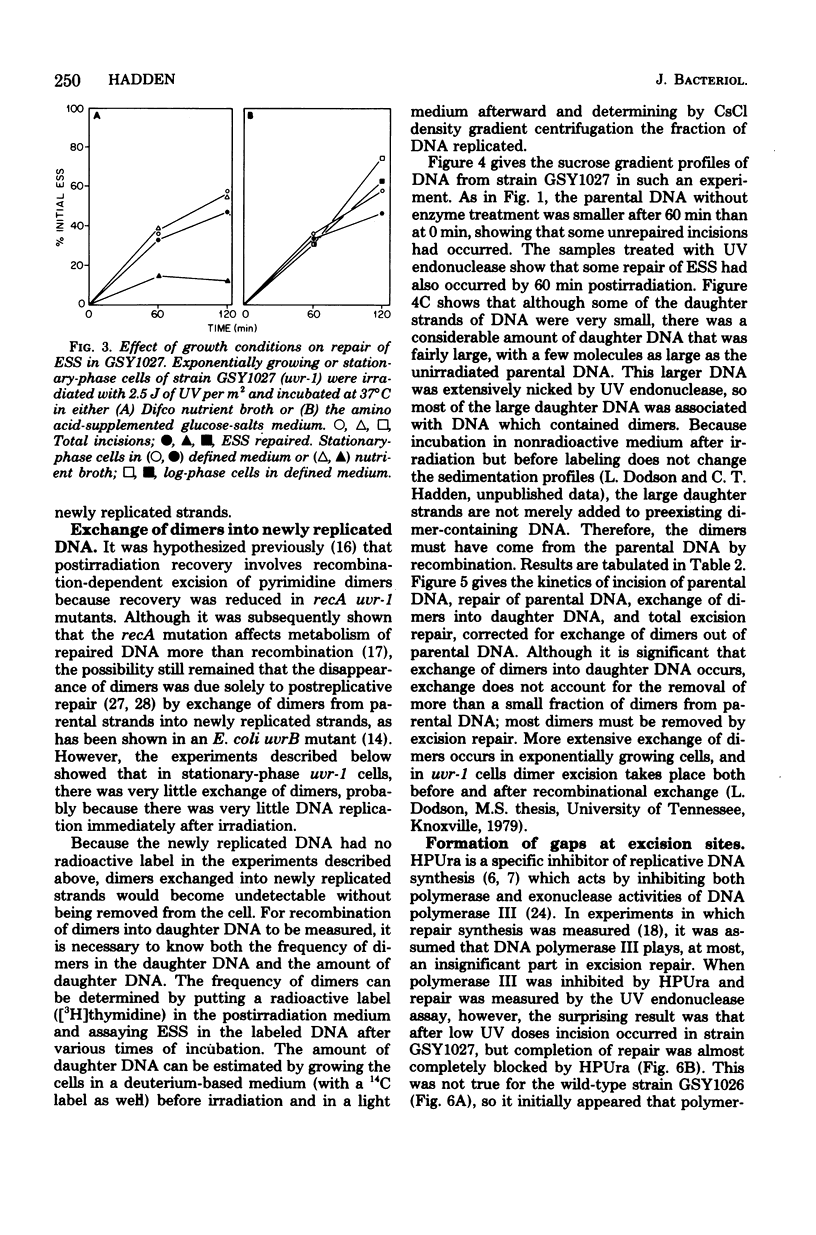
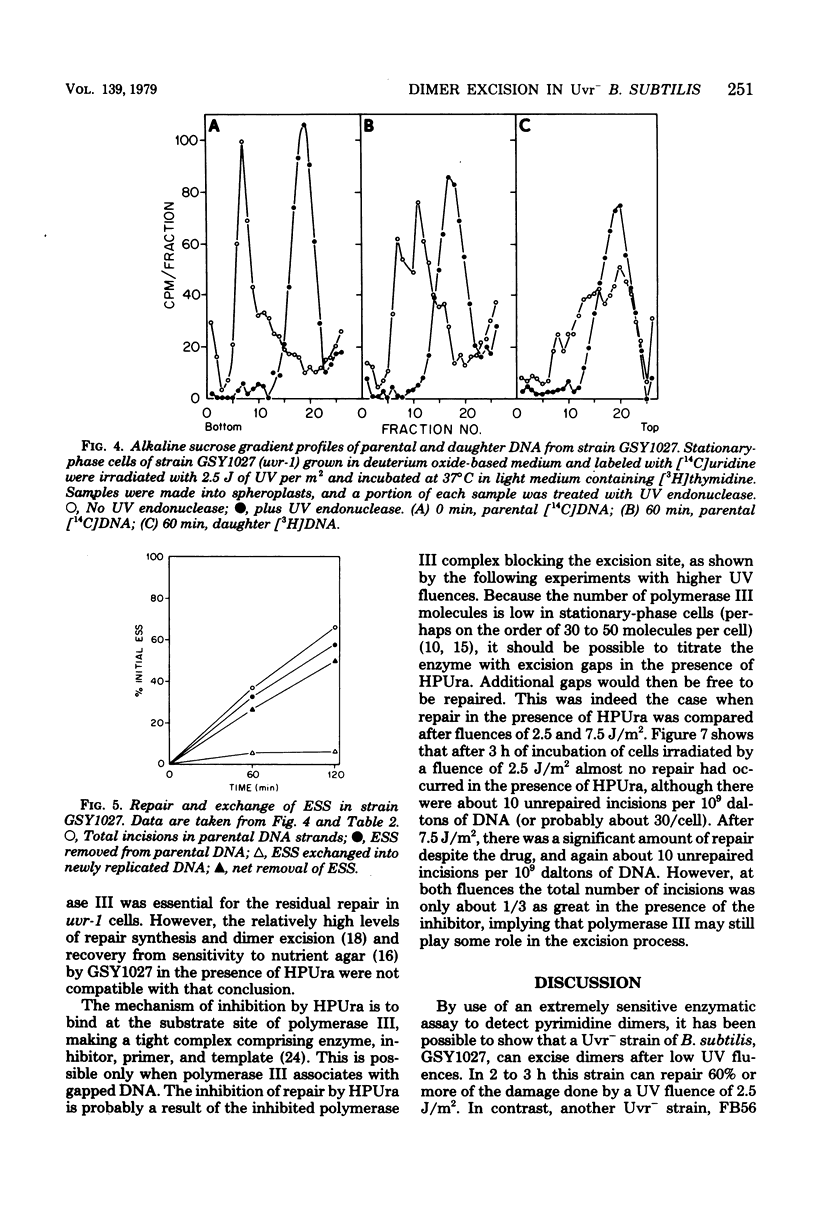
A technique which allows the measurement of small numbers of pyrimidine dimers in the deoxyribonucleic acid (DNA) of cells of Bacillus subtilis irradiated with ultraviolet light has been used to show that a strain mutant at the uvr-1 locus is able to excise pyrimidine dimers. Excision repair in this strain was slow, but incision may not be rate limiting because single-strand breaks in DNA accumulate under some conditions. Excision repair probably accounted for a liquid-holding recovery previously reported to occur in this strain. Recombinational exchange of pyrimidine dimers into newly replicated DNA was readily detected in uvr-1 cells, but this exchange did not account for more than a minor fraction of the dimers removed from parental DNA. Excision repair in the uvr-1 strain was inhibited by a drug which complexes DNA polymerase III with DNA gaps. This inhibition may be limited to a number of sites equal to the number of DNA polymerase III molecules, and it is inferred that large gaps are produced by excision of dimers. Because the uvr-1 mutation specifically interferes with excision of dimers at incision sites, it is concluded that the uvr-1 gene product may be an exonuclease which is essential for efficient dimer excision.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achey P., Billen D. Saturation of dark repair synthesis: accumulation of strand breaks. Biophys J. 1969 May;9(5):647–653. doi: 10.1016/S0006-3495(69)86409-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D., Carreira L. B., Hadden C. T., Silverstein S. J. Evidence suggestive of compartmentalization of deoxyribonucleic acid-synthesizing systems in freeze-treated Bacillus subtilis. J Bacteriol. 1971 Dec;108(3):1250–1256. doi: 10.1128/jb.108.3.1250-1256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Masker W. E. Deoxyribonucleic acid repair in Escherichia coli mutants deficient in the 5'----3' exonuclease activity of deoxyribonucleic acid polymerase I and exonuclease VII. J Bacteriol. 1977 May;130(2):667–675. doi: 10.1128/jb.130.2.667-675.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarrocchi G., Attolini C., Cobianchi F., Riva S., Falaschi A. Modulation of deoxyribonucleic acid polymerase III level during the life cycle of Bacillus subtilis. J Bacteriol. 1977 Sep;131(3):776–783. doi: 10.1128/jb.131.3.776-783.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. Excision-repair in mutants of Escherichia coli deficient in DNA polymerase I and/or its associated 5' leads to 3' exonuclease. Mol Gen Genet. 1977 Jan 7;150(1):1–12. doi: 10.1007/BF02425319. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K. A method for detecting pyrimidine dimers in the DNA of bacteria irradiated with low doses of ultraviolet light. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2753–2756. doi: 10.1073/pnas.70.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K. Persistence of pyrimidine dimers during post-replication repair in ultraviolet light-irradiated Escherichia coli K12. J Mol Biol. 1974 Jul 25;87(1):103–119. doi: 10.1016/0022-2836(74)90563-4. [DOI] [PubMed] [Google Scholar]
- Gass K. B., Cozzarelli N. R. Further genetic and enzymological characterization of the three Bacillus subtilis deoxyribonucleic acid polymerases. J Biol Chem. 1973 Nov 25;248(22):7688–7700. [PubMed] [Google Scholar]
- Hadden C. T. Gap-filling repair synthesis induced by ultraviolet light in a Bacillus subtilis Uvr- mutant. J Bacteriol. 1979 Jul;139(1):239–246. doi: 10.1128/jb.139.1.239-246.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden C. T. Postirradiation recovery dependent on the uvr-1 locus in Bacillus subtilis. J Bacteriol. 1976 Oct;128(1):317–324. doi: 10.1128/jb.128.1.317-324.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden C. T. Repair and subsequent fragmentation of deoxyribonucleic acid in ultraviolet-irradiated Bacillus subtilis recA. J Bacteriol. 1977 Dec;132(3):856–861. doi: 10.1128/jb.132.3.856-861.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Anagnostopoulos C. Chromosomal location and properties of radiation sensitivity mutations in Bacillus subtilis. J Bacteriol. 1970 Aug;103(2):295–301. doi: 10.1128/jb.103.2.295-301.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laipis P. J., Ganesan A. T. A deoxyribonucleic acid polymerase I-deficient mutant of Bacillus subtilis. J Biol Chem. 1972 Sep 25;247(18):5867–5871. [PubMed] [Google Scholar]
- Low R. L., Rashbaum S. A., Cozzarelli N. R. Mechanism of inhibition of Bacillus subtilis DNA polymerase 3 by the arylhydrazinopyrimidine antimicrobial agents. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2973–2977. doi: 10.1073/pnas.71.8.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman R. H., Clark A. J. Defective excision and postreplication repair of UV-damaged DNA in a recL mutant strain of E. coli K-12. Mol Gen Genet. 1977 Oct 24;155(3):267–277. doi: 10.1007/BF00272805. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Strike P. Excision repair of ultraviolet-irradiated deoxyribonucleic acid in plasmolyzed cells of Escherichia coli. J Bacteriol. 1976 Mar;125(3):787–795. doi: 10.1128/jb.125.3.787-795.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Anderson J. A., Barbour S. D. Excision repair properties of isogenic rec mutants of Escherichia coli K-12. J Bacteriol. 1972 Sep;111(3):723–730. doi: 10.1128/jb.111.3.723-730.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins R. J. Endonuclease-sensitive sites in the DNA of irradiated bacteria: a rapid and sensitive assay. Biochim Biophys Acta. 1973 Jun 8;312(1):33–37. doi: 10.1016/0005-2787(73)90049-x. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]


