Abstract
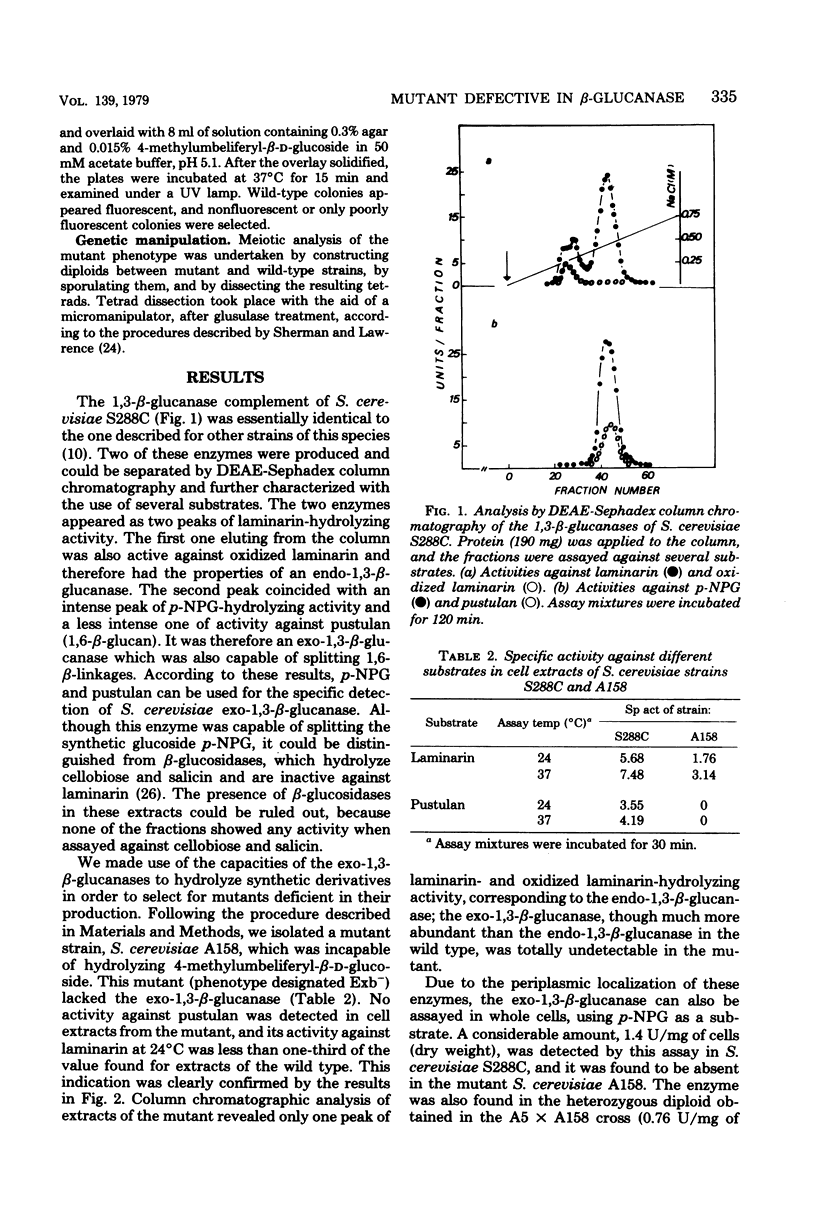
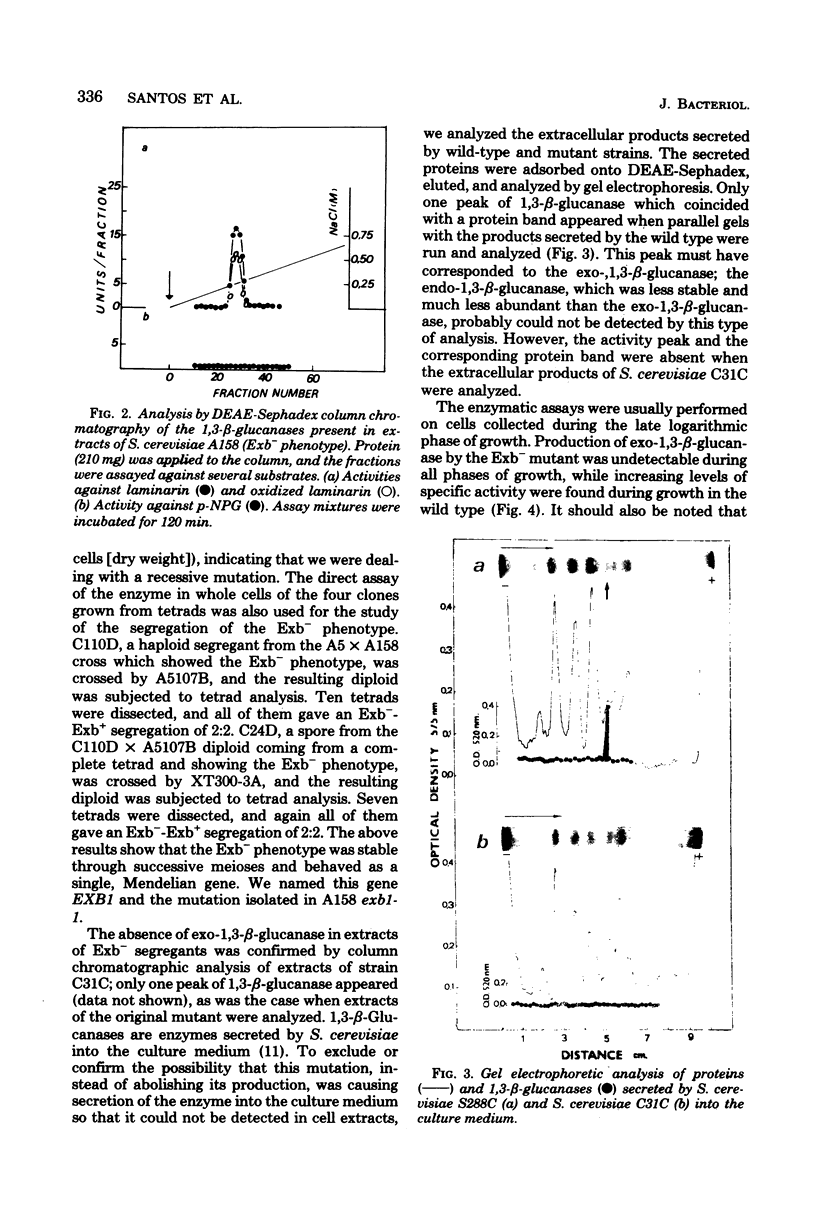
Saccharomyces cerevisiae S288C produced two laminarinases (1,3-beta-glucanases) which were separated by diethylaminoethyl-Sephadex column chromatography; one was an endo-1,3-beta-glucanase, and the other was an exo-1,3-beta-glucanase active not only on laminarin but also on pustulan (1,6-beta-glucan) and on p-nitrophenyl-beta-D-glucoside. A mutant defective in the production of this last enzyme was isolated, and the mutation was named exb1-1. The selection procedure was based on the capacity of exo-1,3-beta-glucanase to hydrolyze synthetic glucosides. The level of endo-1,3-beta-glucanase in cell extracts of the mutant was normal, but the exo-1,3-beta-glucanase could not be detected by column chromatographic analysis of these extracts. The mutant phenotype, recessive in heterozygous diploids, was stable through successive meioses and showed a Mendelian segregation, indicating that the mutation affected a single gene, which was named EXB1. The lack of production of exo-1,3-beta-glucanase persisted through all the phases of growth, but growth itself was not impaired by the enzyme deficiency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A. T., Phaff H. J. Exo-beta-glucanases in yeast. Biochem J. 1968 Sep;109(3):347–360. doi: 10.1042/bj1090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-el-Al A. T., Phaff H. J. Purification and properties of endo-beta-glucanase in the yeast Hanseniaspora valbyensis. Can J Microbiol. 1969 Jul;15(7):697–701. doi: 10.1139/m69-123. [DOI] [PubMed] [Google Scholar]
- Barras D. R. A -glucan endo-hydrolase from Schizosaccharomyces pombe and its role in cell wall growth. Antonie Van Leeuwenhoek. 1972;38(1):65–80. doi: 10.1007/BF02328078. [DOI] [PubMed] [Google Scholar]
- Biely P., Krátký Z., Bauer S. Interaction of concanavalin A with external mannan-proteins of Saccharomyces cerevisiae. Glycoprotein nature of beta-glucanases. Eur J Biochem. 1976 Nov 1;70(1):75–81. doi: 10.1111/j.1432-1033.1976.tb10957.x. [DOI] [PubMed] [Google Scholar]
- Brock T. D. Beta-glucanase of yeast. Biochem Biophys Res Commun. 1965 May 18;19(5):623–629. doi: 10.1016/0006-291x(65)90385-2. [DOI] [PubMed] [Google Scholar]
- Brock T. D. Biochemical and cellular changes occuring during conjugation in Hansenula wingei. J Bacteriol. 1965 Oct;90(4):1019–1025. doi: 10.1128/jb.90.4.1019-1025.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortat M., Matile P., Wiemken A. Isolation of glucanase-containing vesicles from budding yeast. Arch Mikrobiol. 1972;82(3):189–205. doi: 10.1007/BF00412191. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Farkas V., Biely P., Bauer S. Extracellular beta-glucanases of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Sep 15;321(1):246–255. doi: 10.1016/0005-2744(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet G. H., Phaff H. J. Glucanases in Schizosaccharomyces. Isolation and properties of the cell wall-associated beta(1 leads to 3)-glucanases. J Biol Chem. 1974 Mar 25;249(6):1717–1728. [PubMed] [Google Scholar]
- Johnson B. F. Lysis of yeast cell walls induced by 2-deoxyglucose at their sites of glucan synthesis. J Bacteriol. 1968 Mar;95(3):1169–1172. doi: 10.1128/jb.95.3.1169-1172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matile P., Cortat M., Wiemken A., Frey-Wyssling A. Isolation of glucanase-containing particles from budding Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1971 Mar;68(3):636–640. doi: 10.1073/pnas.68.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Santos T., Sanchez M., Villanueva J. R., Nombela C. Regulation of the beta-1,3-glucanase system in Penicillium italicum: glucose repression of the various enzymes. J Bacteriol. 1978 Feb;133(2):465–471. doi: 10.1128/jb.133.2.465-471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle M. A., Halvorson H. O. A comparison of -glucanase and -glucosidase in Saccharomyces lactis. Biochim Biophys Acta. 1971 Oct;250(1):165–171. doi: 10.1016/0005-2744(71)90130-6. [DOI] [PubMed] [Google Scholar]



