Abstract
Two of the enzymes responsible for tryptophan biosynthesis in Bacillus subtilis have been extensively purified. These proteins are indole-3-glycerol phosphate synthase and N-(5'-phosphoribosyl) anthranilate isomerase. By comparison to the non-differentiating enteric bacteria in which these two enzymes are fused into a single polypeptide, the isolation of the indoleglycerol phosphate synthase and phosphoribosyl anthranilate isomerase from B. subtilis has demonstrated that the two proteins are separate species in this organism. The two enzymes were clearly separable by anion-exchange chromatography without any significant loss of activity. Molecular weights were determined for both enzymes by gel filtration and sodium dodecyl sulfate-slab gel electrophoresis, and indicated that the indoleglycerol phosphate synthase is the slightly larger of the two proteins. The minimum molecular weight for indoleglycerol phosphate synthase was 23,500, and that for phosphoribosyl anthranilate isomerase was 21,800. Both enzymes have been examined as to conditions necessary to achieve maximal activity of their individual functions and to maintain that activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
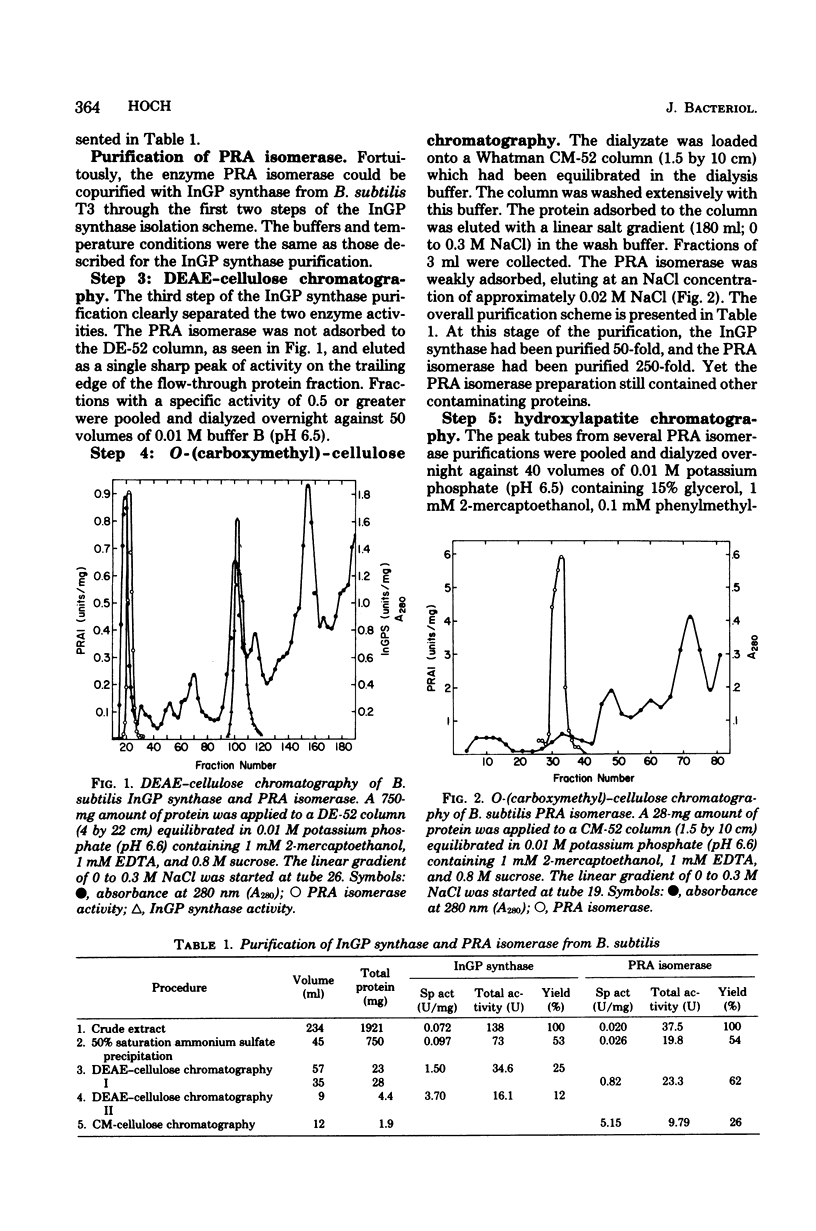
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton B. C., Whitt D. D. The isolation and genetic characterization of mutants of the tryptophan system of Bacillus subtilis. Genetics. 1969 Jul;62(3):445–460. doi: 10.1093/genetics/62.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. N-(5'-phosphoribosyl)anthranilate isomerase-indol-3-ylglycerol phosphate synthetase of tryptophan biosynthesis. Relationship between the two activities of the enzyme from Escherichia coli. Biochem J. 1970 Dec;120(4):699–707. doi: 10.1042/bj1200699. [DOI] [PMC free article] [PubMed] [Google Scholar]
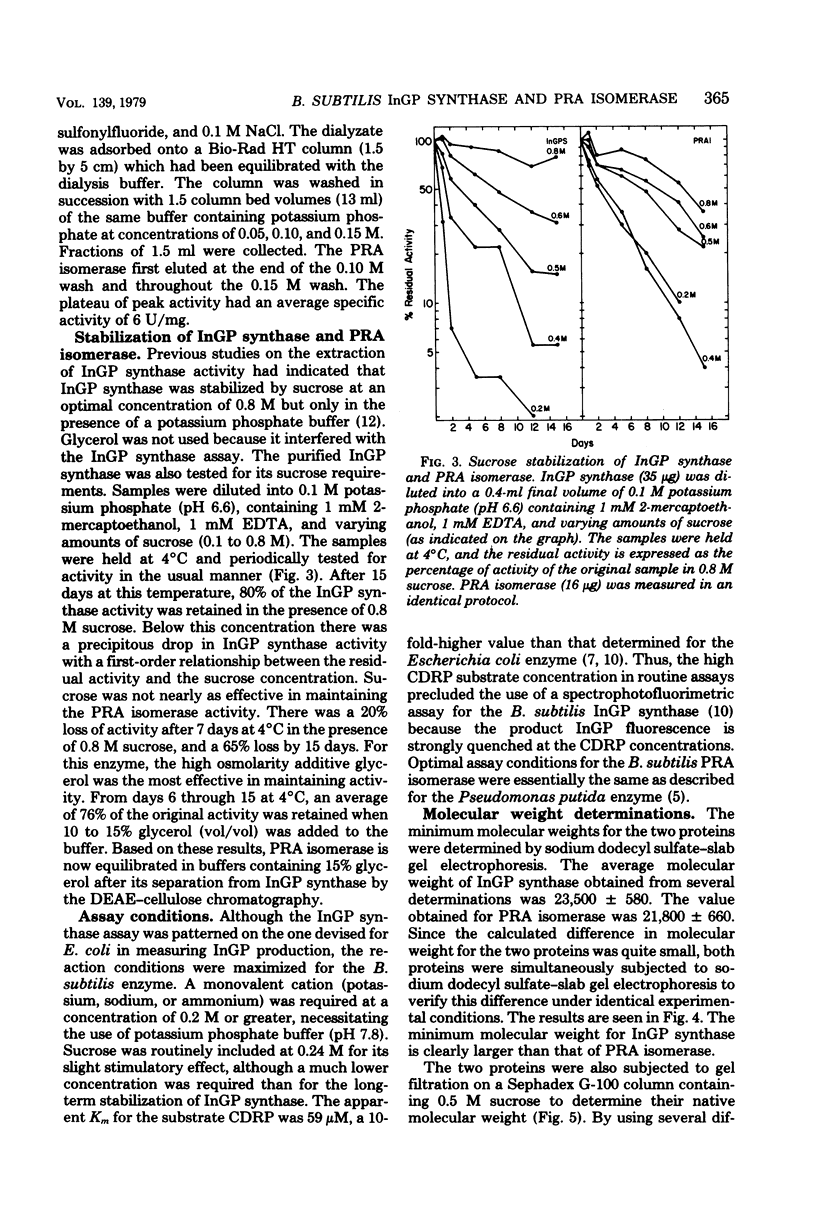
- Creighton T. E., Yanofsky C. Indole-3-glycerol phosphate synthetase of Escherichia coli, an enzyme of the tryptophan operon. J Biol Chem. 1966 Oct 25;241(20):4616–4624. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hankins C. N., Largen M., Mills S. E. A rapid spectrophotofluorometric assay for indoleglycerol phosphate synthase. Anal Biochem. 1975 Dec;69(2):510–517. doi: 10.1016/0003-2697(75)90154-2. [DOI] [PubMed] [Google Scholar]
- Hankins C. N., Mills S. E. A dimer of a single polypeptide chain catalyzes the terminal four reactions of the L-tryptophan pathway in Euglena gracilis. J Biol Chem. 1977 Jan 10;252(1):235–239. [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Crawford I. P. Enzymes of the tryptophan pathway in three Bacillus species. J Bacteriol. 1973 Nov;116(2):685–693. doi: 10.1128/jb.116.2.685-693.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett F. M., DeMoss J. A. Subunit structure of anthranilate synthetase from Neurospora crassa. J Biol Chem. 1975 Sep 10;250(17):6648–6652. [PubMed] [Google Scholar]
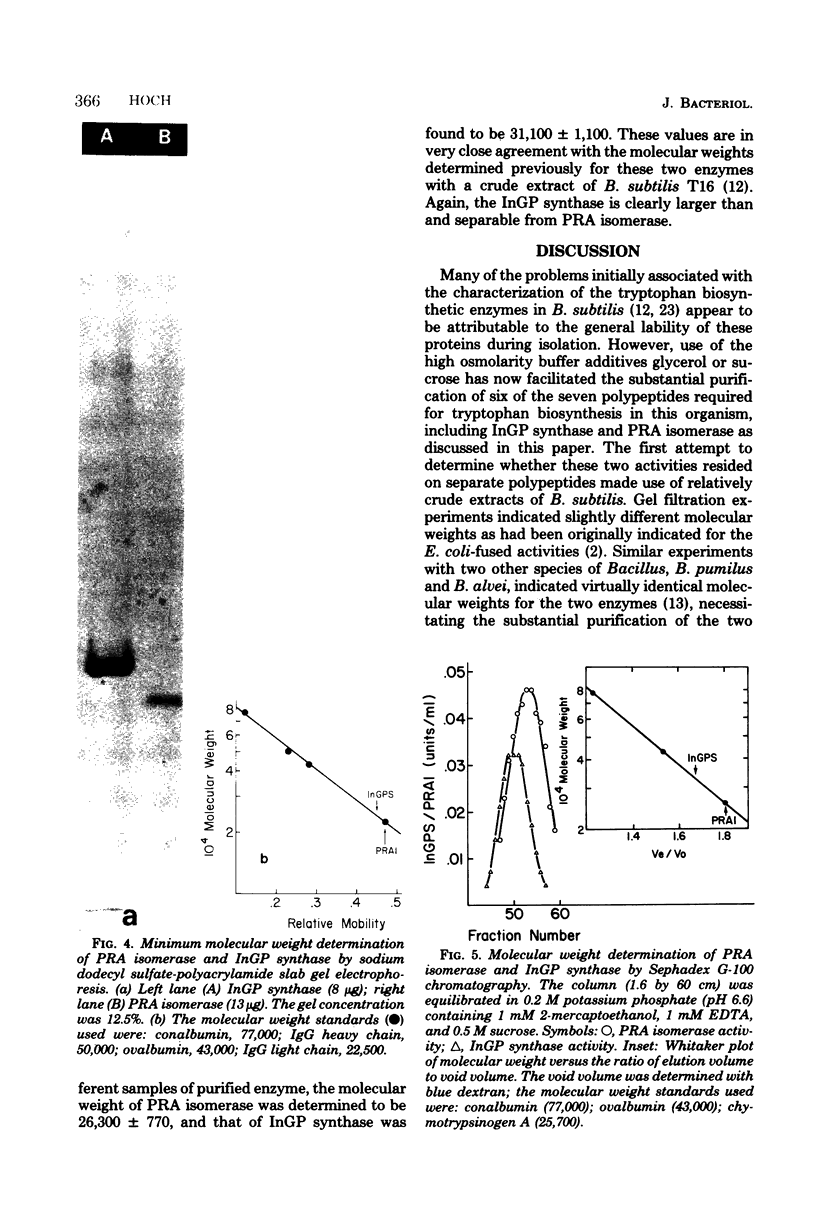
- Kirschner K., Bisswanger H. Multifunctional proteins. Annu Rev Biochem. 1976;45:143–166. doi: 10.1146/annurev.bi.45.070176.001043. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McQuade J. F., 3rd, Creighton T. E. Purification and comparison of the N-(5'-phosphoribosyl)anthranilic acid isomerase-indole-3-glycerol phosphate synthetase of tryptophan biosynthesis from three species of Enterobacteriaceae. Eur J Biochem. 1970 Oct;16(2):199–207. doi: 10.1111/j.1432-1033.1970.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Munoz L., Doi R. H. A procedure to remove protease activities from Bacillus subtilis sporulating cells and their crude extracts. Anal Biochem. 1977 Mar;78(1):165–170. doi: 10.1016/0003-2697(77)90020-3. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt D. D., Carlton B. C. Characterization of mutants with single and multiple defects in the tryptophan biosynthetic pathway in Bacillus subtilis. J Bacteriol. 1968 Oct;96(4):1273–1280. doi: 10.1128/jb.96.4.1273-1280.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Bonner M., Stasiowski S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics. 1971 Dec;69(4):409–433. doi: 10.1093/genetics/69.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]