Abstract
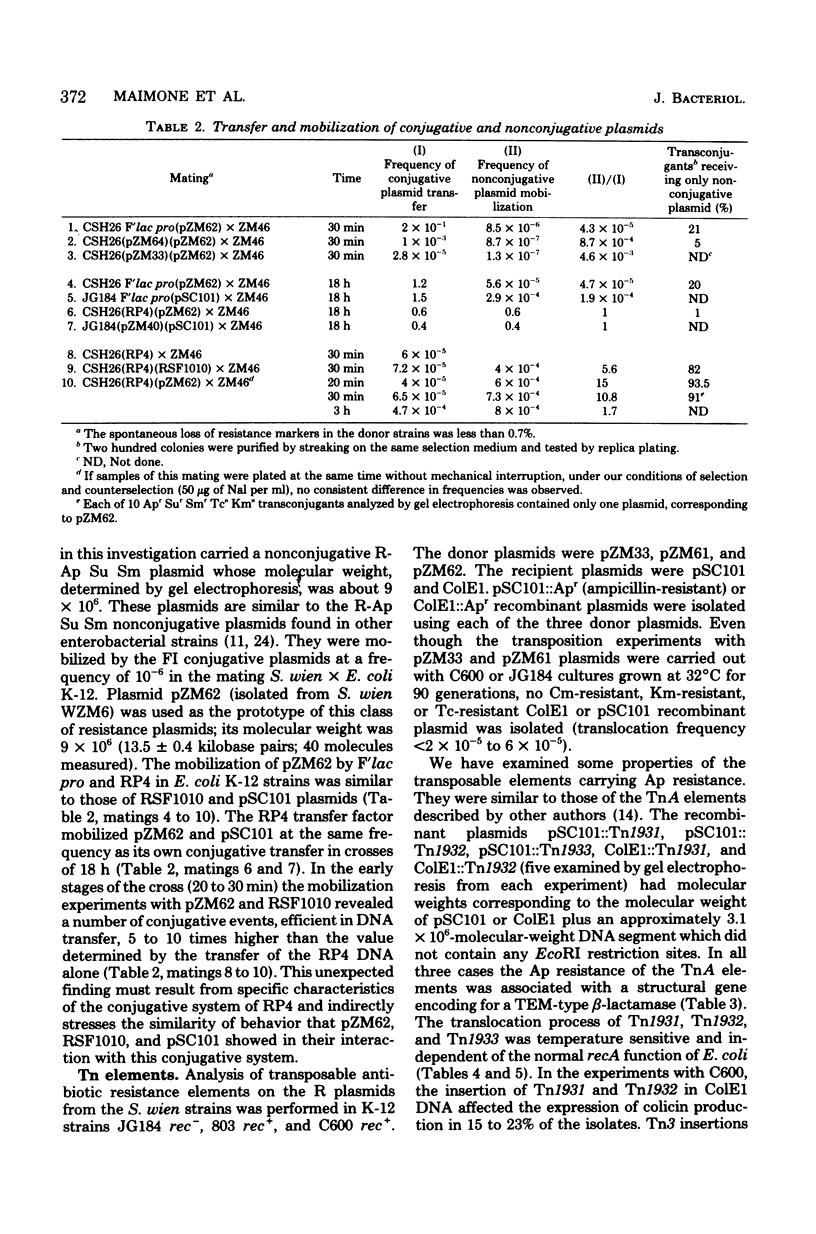
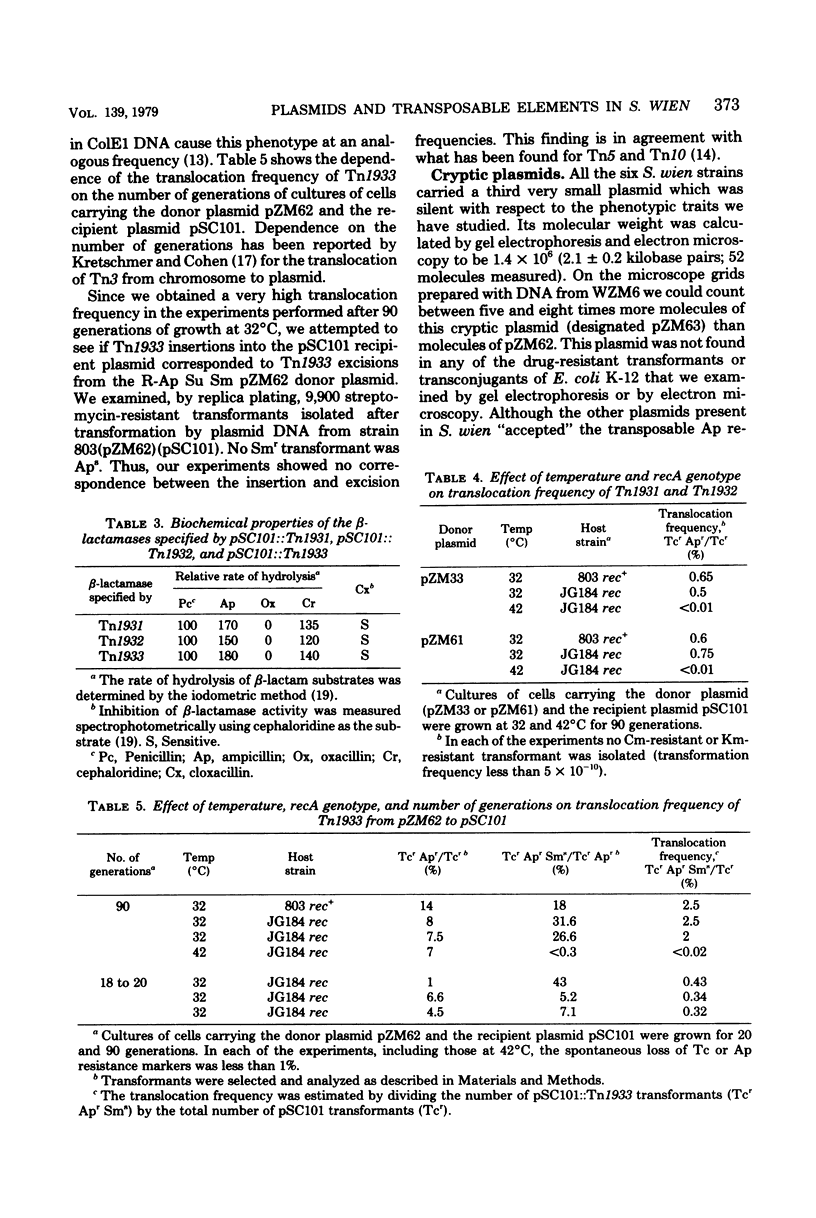
The plasmids from six clinical strains of Salmonella wien have been characterized. All the S. wien strains were found to carry three types of plasmids: an IncFI R-Tc Cm Km Ap (resistance to tetracycline, chloramphenicol, kanamycin, and ampicillin) plasmid, either conjugative or nonconjugative, of large size (90 to 100 megadaltons); an R-Ap Su Sm (resistance to ampicillin, sulfonamide, and streptomycin) plasmid of 9 megadaltons; and a very small (1.4 megadaltons) cryptic plasmid. The characteristics of conjugative R plasmids, recombinant between F'lac pro and the FI nonconjugative plasmid, indicated that regions coding for the donor phenotype were present on this plasmid. The molecular and genetic features of the R plasmids were very close to those described for the R plasmids isolated from S. wien strains of different origin. This fact supported the hypothesis of a clonal distribution of this serotype in Algeria and Europe. The analysis used to identify transposable elements showed the presence of only TnA elements, which were located on both the R-Tc Cm Km Ap and R-Ap Su Sm plasmids. They contained the structural gene for a TEM-type beta-lactamase and had translocation properties analogous to those reported for other TnA's.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S., Threlfall E. J., Carr J. M., McConnell M. M., Smith H. R. Clonal distribution of resistance plasmid-carrying Salmonella typhimurium, mainly in the Middle East. J Hyg (Lond) 1977 Dec;79(3):425–448. doi: 10.1017/s0022172400053286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avril J. L., Dabernat H. J., Gerbaud G. R., Horodniceanu T., Lambert-Zechovsky N., Le Minor S., Mendez B., Chabbert Y. A. Groupes d'incompatibilité des plasmides r chez les souches de Salmonella épidémiques. Ann Microbiol (Paris) 1977 Aug-Sep;128(2):165–175. [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S., Meynell G. G. General method for isolating de-repressed bacterial sex factors. Nature. 1968 Aug 24;219(5156):869–870. doi: 10.1038/219869a0. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Gandini Attardi D., Martini G., Mattoccia E., Tocchini-Valentini G. P. Effect of Xenopus laevis oocyte extract on supercoiled simian virus 40 DNA: formation of complex DNA. Proc Natl Acad Sci U S A. 1976 Feb;73(2):554–558. doi: 10.1073/pnas.73.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: identity of laboratory-constructed plasmids and clinical isolates. J Bacteriol. 1977 Jan;129(1):530–533. doi: 10.1128/jb.129.1.530-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. Incompatibility exhibited by colicin plasmids E1, E2, and E3 in Escherichia coli. J Bacteriol. 1974 Aug;119(2):478–483. doi: 10.1128/jb.119.2.478-483.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. Isolation, mapping, and examination of effects of TnA insertions in ColE1 plasmids. J Bacteriol. 1977 Jan;129(1):482–491. doi: 10.1128/jb.129.1.482-491.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Brevet J., Cohen S. N. Involvement of multiple translocating DNA segments and recombinational hotspots in the structural evolution of bacterial plasmids. J Mol Biol. 1976 Dec;108(2):333–360. doi: 10.1016/s0022-2836(76)80124-6. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Cohen S. N. Site specific recA--independent recombination between bacterial plasmids: involvement of palindromes at the recombinational loci. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1373–1377. doi: 10.1073/pnas.72.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer P. J., Cohen S. N. Selected translocation of plasmid genes: frequency and regional specificity of translocation of the Tn3 element. J Bacteriol. 1977 May;130(2):888–899. doi: 10.1128/jb.130.2.888-899.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Sykes R. B. Properties of the beta-lactamase specified by the Pseudomonas plasmid RPL11. J Bacteriol. 1977 Oct;132(1):341–345. doi: 10.1128/jb.132.1.341-345.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C., Heffron F., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: independence from host rec functions and orientation of insertion. J Bacteriol. 1976 Oct;128(1):425–434. doi: 10.1128/jb.128.1.425-434.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. R., Humphreys G. O., Anderson E. S. Genetic and molecular characterisation of some non-transferring plasmids. Mol Gen Genet. 1974 Mar 27;129(3):229–242. doi: 10.1007/BF00267915. [DOI] [PubMed] [Google Scholar]
- Thompson R., Hughes S. G., Broda P. Plasmid identification using specific endonucleases. Mol Gen Genet. 1974;133(2):141–149. doi: 10.1007/BF00264835. [DOI] [PubMed] [Google Scholar]
- Willshaw G. A., Smith H. R., Anderson E. S. Molecular studies of FIme resistance plasmids, particularly in epidemic Salmonella typhimurium. Mol Gen Genet. 1978 Feb 7;159(1):111–116. doi: 10.1007/BF00401755. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]


