Abstract
Infection with Theiler's murine encephalomyelitis virus (TMEV) in the central nervous system (CNS) causes an immune system-mediated demyelinating disease similar to human multiple sclerosis in susceptible but not resistant strains of mice. To understand the underlying mechanisms of differential susceptibility, we analyzed viral replication, cytokine production, and costimulatory molecule expression levels in microglia and macrophages in the CNS of virus-infected resistant C57BL/6 (B6) and susceptible SJL/J (SJL) mice. Our results indicated that message levels of TMEV, tumor necrosis factor alpha, beta interferon, and interleukin-6 were consistently higher in microglia from virus-infected SJL mice than in those from B6 mice. However, the levels of costimulatory molecule expression, as well as the ability to stimulate allogeneic T cells, were significantly lower in TMEV-infected SJL mice than in B6 mice. In addition, microglia from uninfected naïve mice displayed differential viral replication, T-cell stimulation, and cytokine production, similar to those of microglia from infected mice. These results strongly suggest that different levels of intrinsic susceptibility to TMEV infection, cytokine production, and T-cell activation ability by microglia contribute to the levels of viral persistence and antiviral T-cell responses in the CNS, which are critical for the differential susceptibility to TMEV-induced demyelinating disease between SJL and B6 mice.
BeAn and DA are members of Theiler's original subgroup of Theiler's murine encephalitis virus (TMEV) (52). Intracerebral inoculation of susceptible mice, such as SJL/J (SJL) mice, with either of these viruses results in a biphasic disease characterized by early encephalitis and late chronic demyelination (24). Infection of susceptible mice with these viruses results in a chronic, white matter-demyelinating disease similar to human multiple sclerosis (24). In susceptible strains, infection of the central nervous system (CNS) with TMEV leads to a chronic immune response to viral antigens, which eventually leads to autoimmune responses against myelin autoantigens (29). In contrast, resistant mouse strains, such as C57BL/6 (B6), rapidly clear virus from the CNS and do not develop demyelinating disease, suggesting that viral persistence in these mice corresponds to susceptibility to disease (26, 42, 45). Demyelination in susceptible mice is considered to be immunity mediated, as removal of immune components reduces the clinical onset and severity of demyelinating disease (9, 25, 44, 47).
In particular, infiltration of proinflammatory CD4+ Th1-type cells has been associated with tissue destruction and demyelination (41, 56). A number of CD4+ T cells specific for TMEV during the course of disease in SJL mice recognize four predominant viral capsid epitopes (VP1233-250, VP274-86, VP324-37, and VP451-70), with one each on the external and internal capsid proteins (10, 19, 55, 56). The external capsid epitopes appear to account for the majority (∼80%) of major histocompatibility complex (MHC) class II-restricted T-cell responses to TMEV capsid proteins (55, 57). Recently, viral capsid epitopes recognized by CNS-infiltrating CD4+ T cells from TMEV-infected B6 mice have also been identified (18). When levels of virus capsid-specific CD4+ T cells in the CNS are compared between B6 and SJL mice at early stages of viral infection, significantly higher levels are found in the CNS of resistant B6 mice (30), suggesting that virus-specific CD4+ T cells are important for protection from demyelinating disease during initial immune responses (2). Similarly, levels of CNS-infiltrating virus-specific CD8+ T cells in the CNS are as much as threefold higher in resistant mice at the same time point (28). Therefore, it appears that levels of both initial CD4+ and CD8+ T-cell responses to the virus are critically important in setting the stage of viral persistence/clearance and consequent susceptibility or resistance to inflammatory demyelinating disease.
In order to further understand the potential mechanisms of differences in susceptibility and antiviral immunity levels between SJL and B6 mice, the properties of resident microglial cells and infiltrating macrophages in the CNS during the early stage of viral infection in these mouse strains were investigated. It has previously been established that nonprofessional antigen-presenting cells (APCs; mainly microglial cells and astrocytes) isolated from the CNS of TMEV-infected SJL mice are capable of presenting antigens to both TMEV- and CNS autoantigen-specific T-cell hybridomas and clones (21, 33, 37). Furthermore, microglial cells and/or infiltrating macrophages in the CNS are known to be a major cell population supporting viral persistence during chronic infection (4). Hence, these cells support the replication of TMEV and the activation and/or differentiation of CD4+ and CD8+ T cells infiltrating the CNS of virus-infected mice. Therefore, CNS APCs involved in triggering T-cell responses and harboring viral persistence may ultimately determine susceptibility/resistance to TMEV-IDD via their effects on virus clearance/persistence as well as on target tissue inflammation.
In this study, we compared the potential roles of microglia and macrophages from TMEV-infected susceptible SJL and resistant B6 mice in the innate and adaptive immune responses affecting viral persistence and ultimate disease susceptibility. Our results indicate that viral replication and cytokine production levels are consistently higher in microglia from TMEV-infected SJL mice than in those from B6 mice. In addition, the expression of costimulatory molecules is significantly higher in resistant B6 mice throughout the course of viral infection, suggesting more efficient T-cell activation in resistant B6 mice. On the other hand, both virus replication and type I interferon (IFN) production in microglia from naïve SJL mice are significantly higher than those in such cells from naïve B6 mice. Therefore, differences in the intrinsic properties of microglia in viral replication and virus-induced innate cytokine production are likely to contribute significantly to viral persistence, cellular infiltration to the CNS, and consequent inflammation, leading to the development of demyelinating disease.
MATERIALS AND METHODS
Animals.
Four- to six-week-old female SJL/J, C57BL/6, and BALB/c mice were purchased from Charles River Laboratories via the National Cancer Institute or from Harlan Sprague Dawley (Indianapolis, IN). Experimental procedures with mice were approved by the animal care and use committee of Northwestern University.
Virus and infection.
The BeAn strain of TMEV was propagated in BHK cells grown in Dulbecco's modified Eagle medium supplemented with 7.5% donor calf serum. Approximately 30 μl (1 × 106 PFU) of TMEV was injected into the right hemisphere of 5- to 7-week-old mice anesthetized with sevofluorane or isofluorane.
Synthetic peptides and antibodies.
All synthetic peptides were purchased from Genmed Synthesis (San Francisco, CA). Peptide stocks (2 mM) were prepared in 8% dimethyl sulfoxide in phosphate-buffered saline. All antibodies used for flow cytometry were purchased from BD Pharmingen (San Diego, CA).
Plaque assays.
Brains and spinal cords were removed from mice after perfusion with cold Hanks balanced salt solution and were homogenized using a tissue homogenizer. The tissue homogenate was used to perform standard plaque assays on BHK-21 monolayers as previously described (42). The plaques were visualized after fixation with methanol and staining with 0.1% crystal violet.
Isolation of CNS-infiltrating mononuclear cells.
Sterile Hanks balanced salt solution (30 ml) was used to perfuse the tissues through the left ventricle. Brains and spinal cords were then removed, forced through a steel screen, and incubated at 37°C for 45 min in 250 μg/ml collagenase type 4 (Worthington Biochemical Corp., Lakewood, NJ). A continuous 100% Percoll gradient (Pharmacia, Piscataway, NJ) was used to enrich CNS mononuclear cells after centrifugation at 27,000 × g for 30 min, and the bottom one-third of the gradient was analyzed. CNS-infiltrating microglial cells and macrophages from TMEV-infected mice were isolated from the CNS mononuclear cell preparations by using a MoFlo system (Dako, Fort Collins, CO) following staining with anti-CD11b-allophycocyanin and anti-CD45-phycoerythrin (PE). Isolation of microglia from naïve mice was performed as previously described (8), since uninfected naïve mice do not contain detectable levels of infiltrating macrophages. Briefly, single-cell suspensions of five or six brains and spinal cords were subjected to a discontinuous Percoll gradient (70 and 30%) at 5,000 rpm for 20 min. The cells that accumulated in the interface of the gradient were collected and analyzed by flow cytometry.
Flow cytometry.
CNS-infiltrating lymphocytes were isolated, and Fc receptors were blocked using 50 μl of 2.4G2 hybridoma (ATCC) supernatant by incubation at 4°C for 30 min. Cells were then stained with allophycocyanin-conjugated anti-CD8 (clone Ly-2) and PE-conjugated anti-CD4 (clone L3T4). To visualize macrophage and microglial populations, cells were stained with PE-conjugated anti-CD11b (clone M1/70) and allophycocyanin-conjugated anti-CD45 (clone Ly-5) antibodies. PE-conjugated antibodies to CD80 (clone 16-10A1), CD86 (clone GL1), and CD40 (clone HM40-3) were also used in conjunction with allophycocyanin-labeled anti-CD45 antibody. Intracellular tumor necrosis factor alpha (TNF-α) staining of microglia was performed following incubation with monensin for 6 h according to the manufacturer's instructions, using a Cytofix/Cytoperm kit (BD Biosciences). Cells were analyzed using a Becton Dickenson FACSCalibur flow cytometer. Live cells were gated based on light-scattering properties.
Cytokine/chemokine ELISAs.
Levels of TNF-α, interleukin-6 (IL-6), IP-10 (CXCL10), macrophage chemoattractant protein 1 (MCP-1; CCL2) (BD-Pharmingen, San Diego, CA), and IFN-α (PBL, Piscataway, NJ) were assessed using specific enzyme-linked immunosorbent assay (ELISA) kits. Supernatants from infected microglial and macrophage cultures infected with TMEV (multiplicity of infection [MOI], 10) or mock infected with BHK lysates or medium were assayed for levels of each cytokine/chemokine.
Real-time PCR.
Total RNA was isolated from cells exposed to various treatments, using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized by standard reverse transcription-PCR (RT-PCR) using oligo(dT) primers. Quantitative real-time PCR was carried out with iQ SYBR green supermix (Bio-Rad Laboratories) and the following specific primers, using a Bio-Rad iCycler iQ system (Bio-Rad Laboratories, Hercules, CA): for TMEV, 5′-CCCAGTCCTCAGGAAATGAAGG-3′ and 5′-TCCAAAAGGAGAGGTGCCATAG-3′; for TNF-α, 5′-CTGTGSAGGGAATGGGTGTT-3′ and 5′-GGTCACTGTCCCAGCATCTT-3′; for IL-6, 5′-AGTTGCCTTCTTGGGACTGA-3′ and 5′-TCCACGATTTCCCAGAGAAC-3′; for IFN-α, 5′-ACCTCCTCTGACCCAGGAAG-3′ and 5′-GGCTCTCCAGACTTCTGCTC-3′; for IFN-β, 5′-GGAAAGATTGACGTGGGAGA-3′ and 5′-CTGAGGCATCAACTGACAGG-3′; for IP-10, 5′-AAGTGCTGCCGTCATTTTCT-3′ and 5′-GTGGCAATGATCTCAACACG-3′; for MCP-1, 5′-AAGAGATCAGGGAGTTTGCT-3′ and 5′-CTGCCTCCATCAACCACTTT-3′; and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-AACTTTGGCATTGTGGAAGG-3′ and 5′-ACACATTGGGGGTAGGAACA-3′. Cycle threshold values were normalized to amplification of the housekeeping gene gapdh. Data presented are the values of gene induction over GAPDH levels and are expressed as the means of triplicate analyses with standard deviations.
Measurement of allogeneic T-cell stimulation by CNS APCs.
Isolated splenic CD4+ T cells (3 × 105 cells/well) from BALB/c mice were added to flat-bottomed 96-well microtiter plates (Costar, Cambridge, MA) containing microglia or macrophages in HL-1 medium (Cambrex, Rockland, ME) and then incubated for 72 h. Splenic CD4+ T cells from BALB/c mice were prepared via negative selection, using a mouse T-cell CD4 subset kit (R&D Systems, Minneapolis, MN) as specified by the manufacturer. Briefly, single-splenic-cell suspensions were prepared by gentle teasing through a wire mesh. Cells were incubated with a monoclonal antibody cocktail, and antibody-treated cells were applied to the column to collect the flowthrough cells. The purity of CD4+ T cells was about 92%. CD45int CD11b+ (microglial cells) or CD45hi CD11b+ (macrophages) cells isolated from TMEV-infected B6 and SJL mice were used to stimulate allogeneic BALB/c CD4+ T cells. In some experiments, microglial cells or macrophages from naïve B6 or SJL mice were mock infected or infected with TMEV (MOI, 10) in vitro for 24 h and then used to stimulate allogeneic T cells. Proliferative responses were measured after 72 h of incubation 18 h after the addition of 1 μCi of [3H]TdR per well. Incorporation of [3H]TdR was determined by liquid scintillation counting. Data are plotted as mean cpm ± standard errors of the means for triplicate cultures.
RESULTS
Susceptible mice accumulate significantly lower levels of capsid-specific CD4+ Th1 cells in the CNS than do resistant mice during TMEV infection.
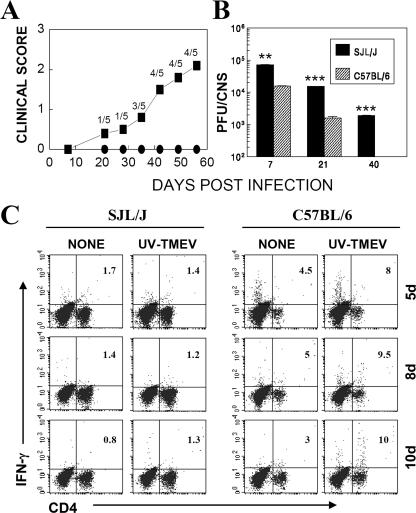
It has previously been well established that SJL mice are extremely susceptible to TMEV-induced demyelinating disease while B6 mice are resistant (26, 42, 45). We first compared susceptibilities, viral persistence, T-cell infiltration, and virus-specific CD4+ T-cell levels in the CNS of TMEV-infected resistant B6 and susceptible SJL mice. Our results confirm previous findings of differential susceptibility and viral persistence in the CNS (Fig. 1A and B) of these mice. Susceptible SJL mice started to develop clinical signs from day 20, with 80% of the population showing clinical signs at day 60, whereas none of the infected B6 mice showed symptoms at either time point (Fig. 1A). Levels of viral persistence in the CNS were consistently higher in susceptible SJL mice than in B6 mice (Fig. 1B). Previous studies have suggested that TMEV-induced disease in SJL mice is a Th1-mediated demyelinating disease (10, 56, 57). Interestingly, levels of specific antiviral Th1 responses in the CNS of SJL mice were significantly lower than those in the CNS of B6 mice, as measured by the intracellular production of IFN-γ upon stimulation of CD4+ T cells with UV-irradiated TMEV (Fig. 1C). These results are in agreement with a recent report comparing levels of TMEV-specific CD4+ T cells between virus-infected SJL and B6 mice (30). Lower levels of virus-specific CD8+ T cells in the CNS of SJL mice than in those of B6 mice were also found (28). Interestingly, the high levels of CD4− cells in the CNS of B6 mice at 5 days postinfection (dpi) (Fig. 1C) represent activated IFN-γ-producing CD8+ T cells (not shown), suggesting again the presence of relatively higher levels of activated CD8+ T cells during the very early stage of TMEV infection in resistant B6 mice than in susceptible SJL mice.
FIG. 1.
Resistant B6 mice have lower viral loads and higher levels of capsid-specific, IFN-γ-producing CD4+ Th1 cells in the CNS during acute infection. (A) Differences in susceptibility to TMEV-induced demyelinating disease between SJL and B6 mice were compared during a time course of 60 days. The clinical score was assessed based on a four-point scale (1 = mild waddling gait, 2 = severe waddling gait and partial hind-limb paralysis, 3 = total hind-limb paralysis, and 4 = forelimb and hind-limb paralysis). (B) Viral persistence levels in the CNS (brains and spinal cords) were determined by plaque assays at 7, 21, and 40 dpi. The levels of viral persistence in the CNS of SJL mice were significantly greater than those in B6 mice. **, P < 0.01; ***, P < 0.001 (based on paired Student's t test). (C) Intracellular cytokine staining was performed on mononuclear cells isolated from the CNS of TMEV-infected mice during early times postinfection (5 to 10 dpi) as described in Materials and Methods. The percentages of IFN-γ-producing CD4+ T cells upon stimulation with phosphate-buffered saline alone (none) or UV-irradiated TMEV (UV-TMEV) are indicated. The percentages of CD4+ T cells among total CNS mononuclear cells were as follows (for SJL and B6 mice, respectively): at 5 dpi, 30.7% and 8.9%; at 8 dpi, 34.4% and 14.4%; and at 10 dpi, 31.5% and 13.9%. The data are representative of three independent experiments.
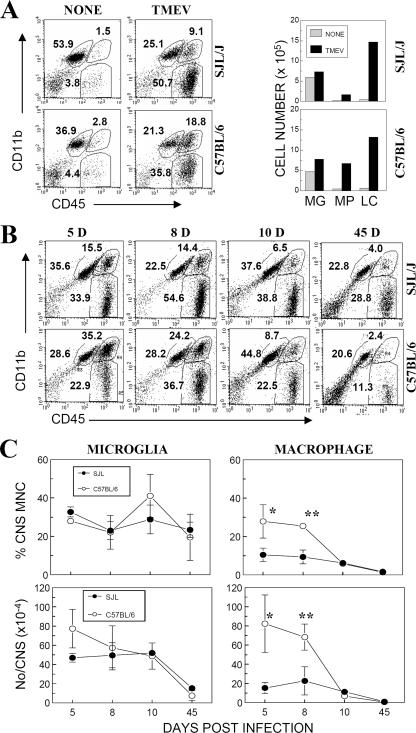
Higher levels of macrophages, but similar levels of microglia, are present in the CNS of resistant mice than in those of susceptible mice.
It has previously been reported that microglial cells/macrophages function as competent APCs and that these cells mainly harbor viral persistence in the CNS of TMEV-infected mice (21, 27). To understand the mechanisms resulting in different levels of antiviral capsid-specific Th1 responses in the CNS of susceptible and resistant mice, we analyzed microglial cells/macrophages accumulated in the CNS of virus-infected mice at 5, 8, 10, and 45 dpi by flow cytometry (Fig. 2). Although the levels of the CD45int CD11b+ population were slightly increased (∼2-fold) following TMEV infection, the levels of the CD45hi CD11b+ and CD45hi CD11b− populations were increased drastically (>10- to 20-fold) in both SJL and B6 mice compared to those in naïve mice (Fig. 2A). These results are consistent with the notion that CD45int cells represent resident microglial cells and CD45hi cells represent infiltrating cells (11, 54). Figure 2B further shows the percentages of mononuclear cell populations in the CNS during the course of TMEV infection. Both susceptible and resistant mice (especially during early acute infection, at 5 to 10 dpi) displayed high levels of CD45int CD11b+ CNS-resident microglial cells (Fig. 2C). The overall number of microglial cells during early infection (5 dpi) appeared to be higher in B6 mice than in SJL mice, but the difference was not statistically significant. In agreement with other CNS inflammation studies (49), the numbers of microglia in both strains remained high during the course of acute TMEV infection. However, CD45hi CD11b+ cell levels in the CNS, containing mainly infiltrating macrophages, were significantly higher (P < 0.01) in resistant B6 mice, particularly at the peak of the acute response, at 8 dpi. These results indicate that relatively lower levels of infiltrating macrophages but similar levels of microglial cells are present in the CNS of susceptible SJL mice compared to those of resistant B6 mice during the early course of infection.
FIG. 2.
Levels of CNS-resident microglial cells and infiltrating mononuclear cells in TMEV-infected SJL and B6 mice. (A) CNS mononuclear cells from naïve SJL and B6 mice (n = 3) or mice infected with TMEV (n = 3) were analyzed at 8 dpi by flow cytometry. Cells were gated on forward and side scatter. The percentages (left) and numbers (right) of CD45int CD11b+, CD45hi CD11b+, and CD45hi CD11b− populations were further analyzed. MG, CD45int CD11b+ microglial cells; MP, CD45hi CD11b+ macrophages; and LC, CD45hi CD11b− leukocytes. (B) SJL and B6 mice were infected with TMEV, and pooled CD45+ microglial cells (CD45int CD11b+) and macrophages (CD45hi CD11b+) from four or five mice were analyzed at 5, 8, 10, and 45 dpi by flow cytometry. Cells were gated as mononuclear cells based on forward and side scatter. One representative experimental result is shown. (C) Percentages and total numbers of CD45int CD11b+ and CD45hi CD11b+ cells in the CNS during the course of TMEV infection in susceptible SJL and resistant B6 mice. The data represent three separate experiments. *, P < 0.05; **, P < 0.001. MNC, mononuclear cells.
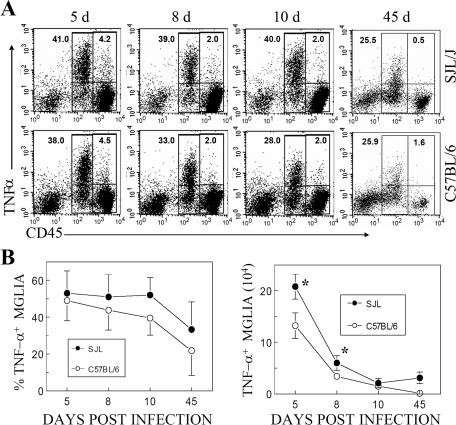
Microglia in both susceptible and resistant TMEV-infected mice are the major source of TNF-α.
We further examined the differences in microglia from these two strains. Since TNF-α levels in the CNS may contribute to protection/pathogenesis of viral demyelinating disease (22), levels of TNF-α production by mononuclear cells isolated at different time points from the CNS of TMEV-infected mice were assessed without further stimulation (Fig. 3). The data show that irrespective of susceptibility, vast majorities of CD45int microglial cells in both strains produced TNF-α (>50% of CD45int cells are TNF-α+ at all times) during the course of TMEV infection. In contrast, a very small proportion of CD45hi CNS mononuclear cells produced TNF-α. These results suggest that microglial cells in the CNS of TMEV-infected mice are highly activated to produce TNF-α and that this cell population is a predominant source of TNF-α among the different CNS mononuclear cell populations (Fig. 3A). The combined enumeration of TNF-α-producing cell numbers in the CNS from three or four independent experiments further demonstrates that TNF-α-producing microglial levels are significantly (P < 0.05) higher in susceptible SJL mice than those in resistant B6 mice at early stages of viral infection (Fig. 3B). No significant level of IFN-γ production by these mononuclear cells was found in either strain, indicating that these cells do not produce IFN-γ spontaneously without further stimulation (data not shown).
FIG. 3.
CNS-resident microglial cells in TMEV-infected susceptible and resistant mice are a major source of TNF-α. CNS mononuclear cells from virus-infected SJL and B6 mice (four or five mice per group) were stained for intracellular TNF-α after a 6-h incubation without any in vitro stimulation. (A) Representative flow cytometric analysis of TNF-α-producing CD45int microglia as well as TNF-α-producing CD45hi infiltrating mononuclear cells (mainly lymphocytes and macrophages). (B) Total numbers per CNS of TNF-α-producing CD45int microglia in TMEV-IDD-susceptible SJL and -resistant B6 mice during the course of infection. The data represent at least three similar experiments.
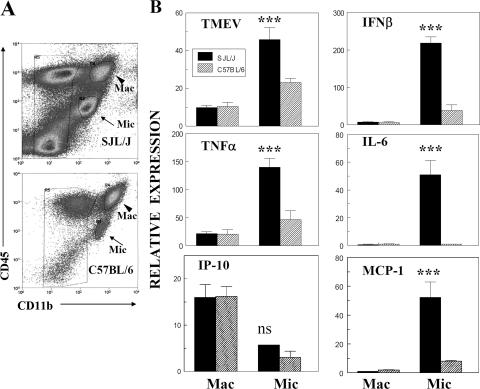
Expression of viral and cytokine message levels is higher in microglia from TMEV-infected susceptible SJL mice.
In order to further investigate potential differences in viral persistence and cytokine production, macrophages and microglial cells were isolated from the CNS of TMEV-infected SJL and B6 mice by flow cytometry, based on CD45 and CD11b expression. Isolated cells were analyzed for levels of viral and cytokine (TNF-α, IFN-β, and IP-10) messages by using quantitative real-time PCR (Fig. 4). All message levels from microglia were significantly higher (P < 0.05), except that for IP-10, which was lower (P < 0.001), than those from macrophages in both susceptible and resistant mice. In addition, these message levels (except that for IP-10) were significantly higher (P < 0.001) in microglia from virus-infected SJL mice than in microglia from virus-infected B6 mice. In contrast, there were no significant differences in all message levels between macrophages from SJL and B6 mice. These results indicate that levels of viral persistence and cytokine production are significantly higher in microglial cells (CD45int) of susceptible mice than in those of resistant mice and that the responses by this cell population are far greater than those by CD45hi cells, containing infiltrating macrophages and highly activated microglial cells.
FIG. 4.
Virus, cytokine, and chemokine message levels expressed in CD45int CD11b+ and CD45hi CD11b+ cell populations in the CNS of TMEV-infected SJL and B6 mice. (A) Flow cytometry of microglial cells (CD45int CD11b+) and macrophages (CD45hi CD11b+) sorted from the CNS of SJL and B6 mice (six mice per group) infected with TMEV at 8 dpi. (B) cDNAs obtained from sorted CNS microglia and infiltrating macrophages were analyzed by quantitative PCR. Relative expression represents x-fold induction of the experimental gene over the GAPDH housekeeping gene level in triplicate reactions. Differences among the groups were analyzed by one-way analysis of variance. Mac, CD45hi CD11b+ population; Mic, CD45int CD11b+ population. All differences in message levels in SJL macrophages versus B6 macrophages are not statistically significant. All differences in message levels in SJL macrophages versus SJL microglia, as well as SJL microglia versus B6 microglia, except for IP-10 levels (not significant), are extremely significant (P < 0.001). Differences in TMEV and IFN-β message levels in B6 macrophages versus B6 microglia are significant (P < 0.05), those in TNF-α levels are not significant, and those in IP-10 levels are extremely significant (P < 0.001).
Viral replication and cytokine production levels are higher in microglial cells and macrophages from naïve SJL mice than in those from B6 mice.
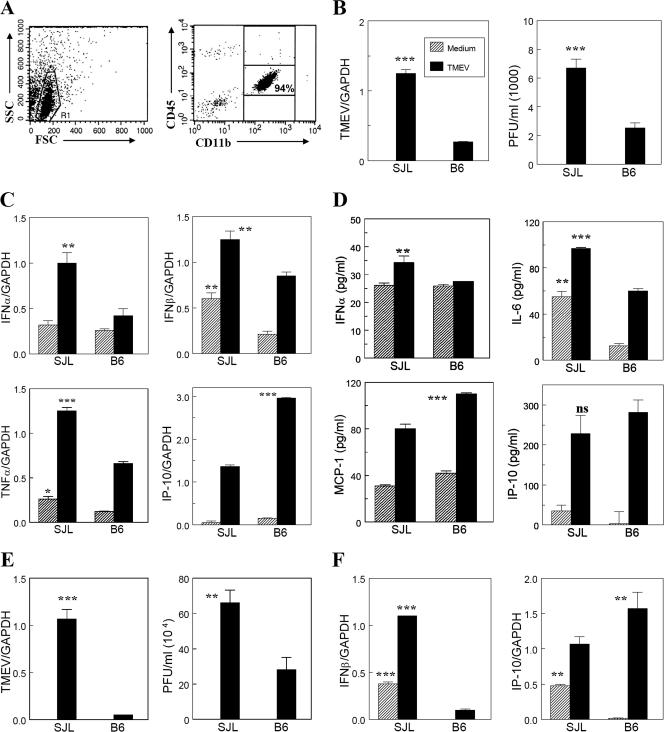
It is conceivable that the elevated levels of viral and cytokine genes in TMEV-infected SJL mice over those in virus-infected B6 mice reflect differential intrinsic properties of microglia from these mice. In order to determine this possibility, isolated microglia from naïve adult SJL and B6 mice were infected with TMEV in vitro, and levels of viral replication and cytokine/chemokine production were assessed (Fig. 5). This isolation procedure yielded >90% of the microglial cells in the viable population (Fig. 5A). It is interesting that TMEV replication, determined by both viral RNA levels and infectious virus production levels, was significantly higher (P < 0.001) in susceptible SJL microglia than in B6 microglia (Fig. 5B). In addition, levels of cytokine genes activated following viral infection were determined by using quantitative RT-PCR (Fig. 5C). Interestingly, basal levels of IFN-β and TNF-α were higher in SJL microglia than in B6 microglia. Levels of IFN-α, IFN-β, and TNF-α gene activation after TMEV infection were also significantly higher in SJL microglia than in B6 microglia. However, IP-10 gene activation levels were significantly higher in B6 microglia. Cytokine levels produced by the microglia were additionally determined by ELISA (Fig. 5D). Although the background level of IL-6 was higher (P < 0.01) in SJL microglia than in B6 microglia (Fig. 5D), TMEV infection significantly (P < 0.01) increased the production of various cytokines and chemokines, similar to the results of RT-PCR; SJL microglia produced significantly higher levels of IFN-α and IL-6 (P < 0.001 and P < 0.01, respectively), whereas B6 microglia produced higher levels of MCP-1 (P < 0.01) and IP-10 (minimally). Since CNS-infiltrating macrophages may also play an important role in viral persistence and/or the pathogenesis of demyelination (4, 30), macrophages from naïve B6 and SJL mice were also compared. Peritoneal macrophages from naïve B6 and SJL mice showed a pattern similar to that of microglia, i.e., higher levels of viral replication (Fig. 5E) and type I IFN production but lower levels of IP-10 (Fig. 5F). These results indicate that both naïve microglial cells and macrophages have intrinsically different levels of susceptibility to TMEV infection and activation of cytokine/chemokine genes upon viral infection between susceptible SJL and resistant B6 mice, which correspond to the patterns observed for the CNS of virus-infected mice.
FIG. 5.
Viral replication and cytokine production upon TMEV infection in isolated microglial cells from the CNS of naïve SJL and B6 mice. microglia were isolated from naïve adult mice by using a Percoll gradient and then were infected with TMEV (MOI, 10) for 24 h to analyze the levels of viral replication and cytokine production. (A) Flow cytometric analysis of isolated microglia. (B) Viral replication levels determined by quantitative real-time PCR and plaque assay. (C) Cytokine/chemokine gene expression levels at 6 h postinfection were assessed by real-time PCR. (D) Cytokine/chemokine levels produced in the culture supernatants by isolated microglia following in vitro infection with TMEV for 24 h were determined by ELISA. (E) Viral replication levels in peritoneal macrophages infected with TMEV (MOI, 10) for 24 h were determined by quantitative real-time PCR and plaque assay. (F) Cytokine/chemokine gene expression levels in peritoneal macrophages were assessed at 6 h postinfection (MOI, 10) by real-time PCR. ***, P < 0.001; **, P < 0.01; and *, P < 0.05.
Microglia of TMEV-infected or naïve resistant B6 mice express higher levels of costimulatory molecules.
It has previously been demonstrated that microglial cells are potent APCs leading to the activation of CD4+ T cells, similar to macrophages (7, 13, 33). Therefore, it is conceivable that the differences in APC function of these cells in SJL and B6 mice may contribute to different levels of TMEV-specific CD4+ T cells in the CNS. In order to examine the possibility of differential expression of MHC class II molecules on these cell populations, which may affect their antigen-presenting function, the surface expression of MHC class II (I-A) molecules on these cells from virus-infected susceptible SJL (H-2s) and resistant B6 (H-2b) mice was compared (not shown). Similar proportions of CNS CD45int microglial cells (20 to 40%) and CD45hi mononuclear cells (40 to 60%) as well as spleen cells (40 to 62%) between both strains expressed similar levels of I-A molecules throughout the course of infection. Hence, the difference in antiviral responses is not likely due to differences in levels of MHC class II expression or the proportions of these class II-expressing CNS APCs.
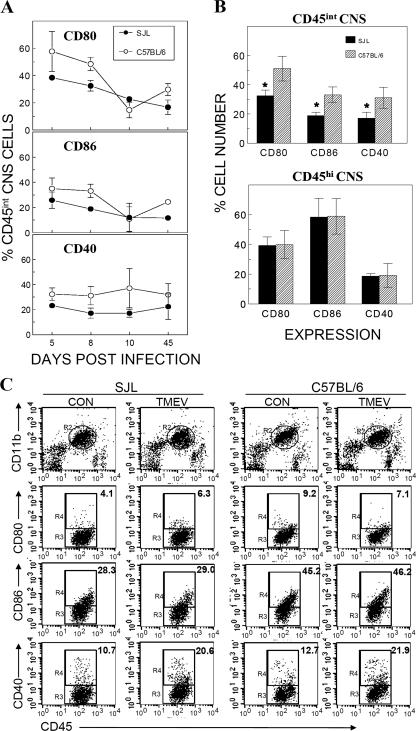
In addition to MHC class II molecules, costimulatory molecules on APCs also play an important role in the activation of antigen-specific CD4+ T cells. In order to understand the mechanisms for differences in antiviral CNS CD4+ T-cell responses between SJL and B6 mice, we examined the expression of three important costimulatory molecules (CD80, CD86, and CD40) on CNS APCs during the course of TMEV infection (Fig. 6A). Compared to those in susceptible SJL mice, higher proportions of CD45int microglial cells in the CNS of resistant B6 mice expressed these costimulatory molecules, especially during early infection. The cumulative data from three separate experiments at 8 dpi clearly indicate that CD45int microglial cells of resistant B6 mice express all of these costimulatory molecules (CD40, CD80, and CD86) in significantly higher (P < 0.05) proportions than those in susceptible SJL mice (Fig. 6B). In contrast, no such differences were seen in the CD45hi population between these two mouse strains. Lower expression levels of costimulatory molecules on SJL microglial cells suggest that inefficient costimulatory signals by this cell population may contribute to the low antiviral Th1 response in susceptible SJL mice.
FIG. 6.
Expression profiles of costimulatory molecules on CNS microglia and infiltrating mononuclear cells of TMEV-infected SJL and B6 mice during the course of viral infection. (A) Expression of costimulatory molecules CD80, CD86, and CD40 on CD45int CNS-resident microglial cells during the course of TMEV infection. The results represent three separate experiments, with three to five mice per group. (B) Cumulative data from three separate experiments showing expression of CD80, CD86, and CD40 on microglia and CNS mononuclear cells from susceptible SJL and resistant B6 mice during early TMEV infection (8 dpi). *, the difference between costimulatory molecule expression at early times between susceptible SJL and resistant B6 mice is statistically significant (P < 0.05). (C) Flow cytometric analysis of costimulatory molecules on isolated microglia from naïve SJL and B6 mice following in vitro infection with TMEV for 24 h.
In order to compare the levels of costimulatory molecules on microglial cells from TMEV-infected mice with those on microglial cells from naïve mice, levels of costimulatory molecules on microglial cells from naïve SJL and B6 mice were assessed by flow cytometry (Fig. 6C). The results clearly indicate that the levels of CD80 and CD86 molecules were significantly higher (2.2- and 1.6-fold, respectively) in microglia from resistant B6 mice than in those from susceptible SJL mice. However, CD40 levels in B6 microglia were not different from those in SJL microglia. The potential induction or suppression of costimulatory molecule expression on microglia following TMEV infection in vitro was further determined. Interestingly, viral infection did not significantly alter the expression of CD80 or CD86 molecules in microglia from both mouse stains, whereas the expression of CD40 increased nearly twofold. These results strongly suggest that different levels of costimulatory molecules on microglial cells from TMEV-infected SJL and B6 mice reflect the differences in intrinsic levels of these molecules on microglia.
Microglia from TMEV-infected susceptible SJL mice induce lower levels of allogeneic T-cell stimulation than do those from resistant B6 mice.
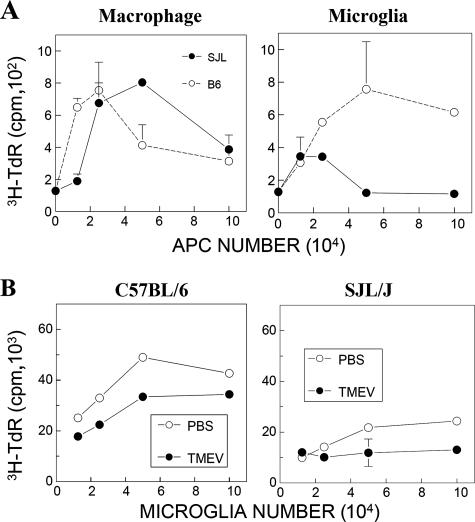
In order to further investigate potential differences in the ability to stimulate T cells between microglia from SJL and B6 mice, we assessed levels of allogeneic T-cell stimulation by isolated microglial cells and microphages from TMEV-infected SJL and B6 mice at 8 dpi (Fig. 7). We chose to utilize an allogeneic system to compare the antigen-presenting functions of these B6 and SJL cells for the identical BALB/c T-cell population. When T-cell proliferation levels were compared, both microglia and macrophages from the CNS of TMEV-infected resistant B6 mice were able to induce more vigorous allogeneic T-cell proliferation than that induced by cells from infected SJL mice (Fig. 7A). These results are consistent with CD4+ and CD8+ T-cell levels in the CNS of susceptible SJL and resistant B6 mice; two- to threefold greater levels are found in resistant B6 mice than in susceptible SJL mice (28, 30).
FIG. 7.
Allogeneic T-cell stimulation by isolated microglia in the CNS of naïve or TMEV-infected SJL and B6 mice. (A) Proliferation of BALB/c T cells upon stimulation with various numbers of isolated macrophages (CD45hi CD11b+) and microglial cells (CD45int CD11b+) from the CNS of TMEV-infected SJL and B6 mice at 8 dpi. Splenic CD4+ T cells (3 × 105 cells/well) from BALB/c mice were cultured with isolated microglia or macrophages for 72 h, and proliferation was then assessed by measuring the levels of [3H]TdR uptake. (B) Proliferation of BALB/c T cells induced by stimulation with various numbers of isolated microglia from naïve SJL and B6 mice. Isolated microglial cells from naïve SJL or B6 mice were infected with control vehicle or TMEV for 24 h, and these cells were used to further stimulate freshly isolated BALB/c splenic CD4+ T cells (3 × 105 cells/well) for 72 h.
We further investigated the possibility that the lower antigen-presenting function of microglia from virus-infected SJL mice reflects intrinsic properties of microglia in SJL and B6 mice. To examine this possibility, microglia were isolated from naïve SJL and B6 mice and then cocultured with isolated CD4+ T cells from naïve BALB/c mice for allogeneic T-cell stimulation (Fig. 7B). Levels of T-cell proliferation stimulated by uninfected B6 microglia were significantly higher (P < 0.01) than those stimulated by SJL microglia. Both SJL and B6 microglia exhibited a significantly reduced ability to stimulate T cells following TMEV infection, although stimulation levels induced by virus-infected B6 microglia were significantly higher than those induced by infected SJL microglia. This pattern is consistent with the higher T-cell-stimulating function displayed by B6 microglia from TMEV-infected mice than that by SJL microglia (Fig. 7A). These results suggest that T-cell responses are more efficiently stimulated by naïve B6 microglia than by SJL microglia, regardless of TMEV infection.
DISCUSSION
We previously demonstrated that levels of TMEV-specific CD8+ T cells in the CNS of virus-infected susceptible SJL mice are far lower than those in resistant B6 mice (28). In addition, levels of CD4+ T cells specific for TMEV capsid epitopes are also significantly lower in the CNS of infected susceptible SJL mice than in those of resistant B6 mice, despite the reverse pattern of overall CD4+ T-cell infiltration (Fig. 1 and data not shown). Low initial levels of antiviral T-cell responses in susceptible mice appear to contribute to the establishment of viral persistence and consequent demyelinating disease. However, the potential mechanisms involved in the differential T-cell responses in these mice are unclear. In particular, the role of resident glial cells in the initiation and/or maintenance of antiviral immune responses is not known. It is conceivable that innate chemokine/cytokine responses to viral infection and the consequent establishment of viral persistence may affect cellular infiltration and subsequent adaptive T-cell responses in the CNS of infected mice.
Our results in this study show that TMEV-infected susceptible SJL mice exhibit similarly high numbers of CD45int CD11b+ cells (microglial cells) and lower numbers of CD45hi CD11b+ cells (mainly macrophages) in the CNS than those of resistant B6 mice during the early stage of infection (5 to 8 dpi) (Fig. 2). Almost all CD45int cells in the CNS of TMEV-infected mice are CD11bhi, which is considered a resident parenchymal microglial population (11, 54). Most CD45hi CD11bhi cells in the CNS of virus-infected mice appear to be infiltrating, bone marrow-derived macrophages/monocytes, although some resident parenchymal microglia are known to become an overlapping CD45hi CD11b+ population during the peak of experimental autoimmune encephalomyelitis (40, 50). For the simplicity of discussion, we refer to CD45int CD11b+ cells as resident microglial cells and CD45hi CD11b+ cells as infiltrating macrophages in this paper. During the course of acute TMEV infection, the numbers of microglial cells and infiltrating macrophages in both strains continue to increase and then decline during late-stage (after 10 days) infection, as seen in other CNS inflammatory diseases (49). Since these cell types are known to be the major reservoir of viral persistence (27), high levels of these cells may provide more target cells for viral infection, resulting in viral persistence. Interestingly, viral persistence and replication in microglia of susceptible SJL mice are significantly (∼3-fold) higher than those in microglia of resistant B6 mice; this population is the major cell type supporting viral replication in the CNS (Fig. 4). Since levels of microglia in the CNS of susceptible and resistant mice are similar to each other, the higher viral loads in the CNS of susceptible SJL mice appear to reflect viral replication levels in microglia of these mice. Although the mechanisms underlying the differential permissiveness to viral infection/replication in microglia from TMEV-infected B6 and SJL mice are not yet known, they appear to reflect the intrinsic properties of cells from different genetic backgrounds, as the microglial cells and macrophages from naïve mice also showed similar differential viral permissiveness/replication (Fig. 5). Furthermore, such differential susceptibility to TMEV infection does not appear to be limited to microglia and macrophages, as similar differential susceptibility was observed between dendritic cells (DCs) from B6 and SJL mice (14).
The innate cytokines and chemokines produced by glial cells may be important for the establishment of initial cellular infiltration and viral persistence. Our results clearly indicate that microglia in the CNS of TMEV-infected susceptible SJL mice produce greater levels of various cytokines and chemokines than do those in the CNS of resistant B6 mice (Fig. 3 and 5). Previously, several groups reported that higher levels of inflammatory cytokines are present in the CNS of susceptible SJL mice than in that of resistant B6 mice (3, 48). However, the function of these inflammatory cytokines and the cells responsible for this production are not yet clear. The administration of recombinant TNF-α beginning prior to viral infection has been shown to reduce demyelination (38). In contrast, the administration of anti-TNF-α antibodies to TMEV-infected susceptible SJL mice confers resistance to the development of demyelinating disease (15). These results suggest that the temporal presence of TNF-α in conjunction with the time of viral infection may provide either protection or pathogenesis. Thus, the elevated levels of this cytokine produced by microglia in the CNS of virus-infected susceptible mice (Fig. 3 and 5) may contribute to the development of TMEV-induced demyelinating disease. Perhaps the presence of TNF-α may indirectly activate adjacent naïve glial cells to promote viral infection and replication. In addition, TNF-α accumulated in the CNS may also cause endothelial cell activation, promoting cellular infiltration into the CNS and cellular apoptosis, leading to tissue damage following viral infection (16, 39).
Similarly, the levels of IL-6 in the CNS of TMEV-infected SJL mice are also higher than those in infected B6 mice (3), and thus it is most likely that microglia also contribute to the differential levels of IL-6 in the CNS during the early stage of viral infection (Fig. 3 and 5). The potential function of IL-6 in TMEV-IDD infection is not yet clear. It was previously shown that administration of recombinant IL-6 can be protective during early but not later stages of viral infection (46). Interestingly, higher levels of both TNF-α and IL-6 were found in mice resistant to the encephalitis induced by Semliki Forest virus (31), which is opposite to the case for TMEV. The nature of diseases induced by these viruses are different; Semliki Forest virus induces acute encephalitis, which is mainly due to the tissue damage by accompanying high viral loads, and TMEV induces chronic immunemediated demyelinating disease. Therefore, it is conceivable that cytokine functions may be different depending on the nature of the disease and the type of immune cells involved in the disease process.
Direct activation of various cytokine and chemokine genes in microglia upon TMEV infection was previously reported (33, 35). However, no direct comparison of the variety of cytokines induced in microglia upon TMEV infection between susceptible SJL and resistant B6 mice, in conjunction with their APC function and viral persistence, has been reported. It is interesting that microglia from TMEV-infected susceptible SJL mice and naïve SJL microglia following in vitro infection produced significantly higher levels of type I IFNs than those produced by microglia from resistant B6 mice (Fig. 4 and 5). This was highly unexpected, as this cytokine is known to be a potent antiviral agent and TMEV infection is known to inhibit or delay the activation of type I IFN genes via virus leader proteins in L292 cells and primary astrocytes, respectively (36, 53). Furthermore, type I IFN is being utilized as an efficient therapeutic treatment for multiple sclerosis (1, 43). IFNs are also known to exert powerful immunosuppressive functions by downregulating inflammatory T-cell responses (20). Therefore, it is conceivable that delayed production of type I IFNs in TMEV-infected microglia may not be efficient in controlling viral replication but may exert powerful anti-inflammatory immune functions which are critical for viral clearance. This possibility is consistent with our results indicating that TMEV infection stimulates higher levels of type I IFNs, higher levels of viral persistence, and lower levels of antiviral T-cell responses in the CNS of susceptible SJL mice than in the CNS of resistant B6 mice.
Activated resident glial cells, including astrocytes and microglial cells, are capable of processing and presenting TMEV antigens or CNS autoantigens to specific T-cell hybridomas and cell lines (21, 33, 37). In particular, microglial cells and macrophages may play an important role as APCs, because these cells are known to harbor viral persistence and directly influence the activation and/or differentiation of local CD4+ and CD8+ T cells in the CNS (27, 34). T-cell activation signals are provided by cognate interaction of their T-cell receptors with MHC-peptide complexes and by noncognate engagements of costimulatory molecules, such as CD80, CD86, and CD40 (12, 17, 23). These costimulatory molecules are essential for the generation, expansion, and maintenance of CD4+ and CD8+ T-cell responses (12). Interestingly, the blockade of CD80/CD86-CD28 costimulatory interactions during early TMEV infection exacerbates disease severity in susceptible SJL mice (32). In addition, CD40-CD40L interactions are critical for the generation of anti-TMEV cytotoxic T-lymphocyte (CTL) responses (6). It has been shown previously that TMEV infection directly induces various cytokines and the upregulation of costimulatory molecules (33, 35). In addition, there are robust levels of IFN-γ in the CNS of TMEV-infected mice (3, 48), which are presumably produced by activated T cells. Therefore, it is not surprising to see such upregulation of costimulatory molecules on the microglia and macrophages from the CNS of infected mice or following viral infection in vitro. Our data show that the expression of CD80, CD86, and CD40 costimulatory molecules on the microglia of susceptible mice is significantly lower in the CNS of TMEV-infected susceptible SJL mice than in the CNS of resistant B6 mice during early infection (Fig. 6). This difference in costimulatory molecule expression levels appears to be an intrinsic property of microglia in these mice (Fig. 6C). During early infection, the level of costimulatory molecule expression is likely to affect the induction of immune responses to viral epitopes which are necessary for viral clearance, as the ability of SJL microglia to stimulate T cells is significantly lower than that of B6 microglia (Fig. 7). These studies support the notion that lower levels of costimulatory molecules on CNS cells in susceptible SJL mice may contribute to the poor generation of early antiviral T-cell responses compared to that in resistant B6 mice. It is interesting that the expression of costimulatory molecules on TMEV-infected SJL DCs is also significantly lower than that on B6 DCs (14). Consequently, early viral clearance may be delayed and viral persistence may be established in the CNS, leading to the development of demyelinating disease.
Our results demonstrate for the first time that there are differential innate cytokine responses and viral infectivity/replication in microglia and macrophages between susceptible SJL and resistant B6 mice. In addition, high levels of some innate responses to viral infection may not necessarily be beneficial for the host, as vigorously responding glial cells from susceptible mice poorly stimulate T-cell activation. Differences in viral infection/replication and innate cytokine responses in glial cells may provide important contributions to cellular infiltration in the CNS, consequent adaptive antiviral T-cell responses, and retention of inflammatory cells, which are critical for the pathogenesis of TMEV-induced demyelinating disease. These differential responses to viral infection by glial cells appear to reflect intrinsic properties of naïve glial cells of resistant and susceptible mice. However, it is unclear which factors and/or genetic traits determine these differential responses to the virus. It is conceivable that the expression and/or affinity of receptors specific for TMEV may be different between these mouse strains and that this difference may result in differences in viral infectivity/persistence and innate cytokine responses. In addition, the cellular machinery involved in innate immunity, such as the level and/or type of Toll-like receptors, which affect viral infectivity and/or cytokine/chemokine production following viral infection (5, 51), may be different between susceptible SJL and resistant B6 mice. Nevertheless, a further understanding of the mechanisms of the contributions by glial cells to viral replication and consequent CNS inflammatory disease would be very important for advances in the treatment and/or prevention of virally induced CNS diseases.
Acknowledgments
This work was supported by U.S. Public Health Service grants RO1 NS28752, RO1 NS33008, and PO1 NS23349 and by a grant from the National Multiple Sclerosis Society (RG 3392-A5).
Footnotes
Published ahead of print on 22 August 2007.
REFERENCES
- 1.Bayas, A., and R. Gold. 2003. Lessons from 10 years of interferon beta-1b (Betaferon/Betaseron) treatment. J. Neurol. 250(Suppl. 4):IV3-IV8. [DOI] [PubMed] [Google Scholar]
- 2.Borrow, P., C. J. Welsh, and A. A. Nash. 1993. Study of the mechanisms by which CD4+ T cells contribute to protection in Theiler's murine encephalomyelitis. Immunology 80:502-506. [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, J. R., E. Zaczynska, C. D. Katsetos, C. D. Platsoucas, and E. L. Oleszak. 2000. Differential expression of TGF-beta, IL-2, and other cytokines in the CNS of Theiler's murine encephalomyelitis virus-infected susceptible and resistant strains of mice. Virology 278:346-360. [DOI] [PubMed] [Google Scholar]
- 4.Clatch, R. J., S. D. Miller, R. Metzner, M. C. Dal Canto, and H. L. Lipton. 1990. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler's murine encephalomyelitis virus (TMEV). Virology 176:244-254. [DOI] [PubMed] [Google Scholar]
- 5.Dahlberg, A., M. R. Auble, and T. M. Petro. 2006. Reduced expression of IL-12 p35 by SJL/J macrophages responding to Theiler's virus infection is associated with constitutive activation of IRF-3. Virology 353:422-432. [DOI] [PubMed] [Google Scholar]
- 6.Drescher, K. M., L. J. Zoecklein, K. D. Pavelko, C. Rivera-Quinones, D. Hollenbaugh, and M. Rodriguez. 2000. CD40L is critical for protection from demyelinating disease and development of spontaneous remyelination in a mouse model of multiple sclerosis. Brain Pathol. 10:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford, A. L., E. Foulcher, F. A. Lemckert, and J. D. Sedgwick. 1996. Microglia induce CD4 T lymphocyte final effector function and death. J. Exp. Med. 184:1737-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford, A. L., A. L. Goodsall, W. F. Hickey, and J. D. Sedgwick. 1995. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 154:4309-4321. [PubMed] [Google Scholar]
- 9.Friedmann, A., G. Frankel, Y. Lorch, and L. Steinman. 1987. Monoclonal anti-I-A antibody reverses chronic paralysis and demyelination in Theiler's virus-infected mice: critical importance of timing of treatment. J. Virol. 61:898-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerety, S. J., M. K. Rundell, M. C. Dal Canto, and S. D. Miller. 1994. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J. Immunol. 152:919-929. [PubMed] [Google Scholar]
- 11.Greter, M., F. L. Heppner, M. P. Lemos, B. M. Odermatt, N. Goebels, T. Laufer, R. J. Noelle, and B. Becher. 2005. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 11:328-334. [DOI] [PubMed] [Google Scholar]
- 12.Grewal, I. S., and R. A. Flavell. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111-135. [DOI] [PubMed] [Google Scholar]
- 13.Hickey, W. F., and H. Kimura. 1988. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239:290-292. [DOI] [PubMed] [Google Scholar]
- 14.Hou, W., E.-Y. So, and B. S. Kim. 2007. Role of dendritic cells in differential susceptibility to viral demyelinating disease. PLoS Pathogens 3:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue, A., C. S. Koh, H. Yahikozawa, N. Yanagisawa, H. Yagita, Y. Ishihara, and B. S. Kim. 1996. The level of tumor necrosis factor-alpha producing cells in the spinal cord correlates with the degree of Theiler's murine encephalomyelitis virus-induced demyelinating disease. Int. Immunol. 8:1001-1008. [DOI] [PubMed] [Google Scholar]
- 16.Jelachich, M. L., and H. L. Lipton. 1999. Restricted Theiler's murine encephalomyelitis virus infection in murine macrophages induces apoptosis. J. Gen. Virol. 80:1701-1705. [DOI] [PubMed] [Google Scholar]
- 17.June, C. H., J. A. Bluestone, L. M. Nadler, and C. B. Thompson. 1994. The B7 and CD28 receptor families. Immunol. Today 15:321-331. [DOI] [PubMed] [Google Scholar]
- 18.Kang, B., H. K. Kang, and B. S. Kim. 2005. Identification of capsid epitopes of Theiler's virus recognized by CNS-infiltrating CD4+ T cells from virus-infected C57BL/6 mice. Virus Res. 108:57-61. [DOI] [PubMed] [Google Scholar]
- 19.Kang, B. S., M. A. Lyman, and B. S. Kim. 2002. The majority of infiltrating CD8+ T cells in the central nervous system of susceptible SJL/J mice infected with Theiler's virus are virus specific and fully functional. J. Virol. 76:6577-6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaser, A., and H. Tilg. 2001. Interferon-alpha in inflammation and immunity. Cell Mol. Biol. (Noisy-le-Grand) 47:609-617. [PubMed] [Google Scholar]
- 21.Katz-Levy, Y., K. L. Neville, A. M. Girvin, C. L. Vanderlugt, J. G. Pope, L. J. Tan, and S. D. Miller. 1999. Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler's virus-infected mice. J. Clin. Investig. 104:599-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, B. S., J. P. Palma, D. Kwon, and A. C. Fuller. 2005. Innate immune response induced by Theiler's murine encephalomyelitis virus infection. Immunol. Res. 31:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 24.Lipton, H. L., and M. C. Dal Canto. 1976. Chronic neurologic disease in Theiler's virus infection of SJL/J mice. J. Neurol. Sci. 30:201-207. [DOI] [PubMed] [Google Scholar]
- 25.Lipton, H. L., and M. C. Dal Canto. 1976. Theiler's virus-induced demyelination: prevention by immunosuppression. Science 192:62-64. [DOI] [PubMed] [Google Scholar]
- 26.Lipton, H. L., J. Kratochvil, P. Sethi, and M. C. Dal Canto. 1984. Theiler's virus antigen detected in mouse spinal cord 2 1/2 years after infection. Neurology 34:1117-1119. [DOI] [PubMed] [Google Scholar]
- 27.Lipton, H. L., A. S. Kumar, and M. Trottier. 2005. Theiler's virus persistence in the central nervous system of mice is associated with continuous viral replication and a difference in outcome of infection of infiltrating macrophages versus oligodendrocytes. Virus Res. 111:214-223. [DOI] [PubMed] [Google Scholar]
- 28.Lyman, M. A., J. Myoung, M. Mohindru, and B. S. Kim. 2004. Quantitative, not qualitative, differences in CD8+ T cell responses to Theiler's murine encephalomyelitis virus between resistant C57BL/6 and susceptible SJL/J mice. Eur. J. Immunol. 34:2730-2739. [DOI] [PubMed] [Google Scholar]
- 29.Miller, S. D., C. L. Vanderlugt, W. S. Begolka, W. Pao, R. L. Yauch, K. L. Neville, Y. Katz-Levy, A. Carrizosa, and B. S. Kim. 1997. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 3:1133-1136. [DOI] [PubMed] [Google Scholar]
- 30.Mohindru, M., B. Kang, and B. S. Kim. 2006. Initial capsid-specific CD4+ T cell responses protect against Theiler's murine encephalomyelitis virus-induced demyelinating disease. Eur. J. Immunol. 36:2106-2115. [DOI] [PubMed] [Google Scholar]
- 31.Mokhtarian, F., S. L. Wesselingh, S. Choi, A. Maeda, D. E. Griffin, R. A. Sobel, and D. Grob. 1996. Production and role of cytokines in the CNS of mice with acute viral encephalomyelitis. J. Neuroimmunol. 66:11-22. [DOI] [PubMed] [Google Scholar]
- 32.Neville, K. L., M. C. Dal Canto, J. A. Bluestone, and S. D. Miller. 2000. CD28 costimulatory blockade exacerbates disease severity and accelerates epitope spreading in a virus-induced autoimmune disease. J. Virol. 74:8349-8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson, J. K., A. M. Girvin, and S. D. Miller. 2001. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler's virus. J. Virol. 75:9780-9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson, J. K., and S. D. Miller. 2004. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 173:3916-3924. [DOI] [PubMed] [Google Scholar]
- 35.Palma, J. P., and B. S. Kim. 2001. Induction of selected chemokines in glial cells infected with Theiler's virus. J. Neuroimmunol. 117:166-170. [DOI] [PubMed] [Google Scholar]
- 36.Palma, J. P., D. Kwon, N. A. Clipstone, and B. S. Kim. 2003. Infection with Theiler's murine encephalomyelitis virus directly induces proinflammatory cytokines in primary astrocytes via NF-kappaB activation: potential role for the initiation of demyelinating disease. J. Virol. 77:6322-6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palma, J. P., R. L. Yauch, S. Lang, and B. S. Kim. 1999. Potential role of CD4+ T cell-mediated apoptosis of activated astrocytes in Theiler's virus-induced demyelination. J. Immunol. 162:6543-6551. [PubMed] [Google Scholar]
- 38.Paya, C. V., P. J. Leibson, A. K. Patick, and M. Rodriguez. 1990. Inhibition of Theiler's virus-induced demyelination in vivo by tumor necrosis factor alpha. Int. Immunol. 2:909-913. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier, R. P., R. G. Ohye, A. Vanbuskirk, D. D. Sedmak, P. Kincade, R. M. Ferguson, and C. G. Orosz. 1992. Importance of endothelial VCAM-1 for inflammatory leukocytic infiltration in vivo. J. Immunol. 149:2473-2481. [PubMed] [Google Scholar]
- 40.Ponomarev, E. D., L. P. Shriver, K. Maresz, and B. N. Dittel. 2005. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 81:374-389. [DOI] [PubMed] [Google Scholar]
- 41.Pope, J. G., W. J. Karpus, C. VanderLugt, and S. D. Miller. 1996. Flow cytometric and functional analyses of central nervous system-infiltrating cells in SJL/J mice with Theiler's virus-induced demyelinating disease. Evidence for a CD4+ T cell-mediated pathology. J. Immunol. 156:4050-4058. [PubMed] [Google Scholar]
- 42.Pullen, L. C., S. H. Park, S. D. Miller, M. C. Dal Canto, and B. S. Kim. 1995. Treatment with bacterial LPS renders genetically resistant C57BL/6 mice susceptible to Theiler's virus-induced demyelinating disease. J. Immunol. 155:4497-4503. [PubMed] [Google Scholar]
- 43.Rio, J., and X. Montalban. 2005. Interferon-beta 1b in the treatment of multiple sclerosis. Expert Opin. Pharmacother. 6:2877-2886. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez, M., W. P. Lafuse, J. Leibowitz, and C. S. David. 1986. Partial suppression of Theiler's virus-induced demyelination in vivo by administration of monoclonal antibodies to immune-response gene products (Ia antigens). Neurology 36:964-970. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez, M., J. L. Leibowitz, and P. W. Lampert. 1983. Persistent infection of oligodendrocytes in Theiler's virus-induced encephalomyelitis. Ann. Neurol. 13:426-433. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez, M., K. D. Pavelko, C. W. McKinney, and J. L. Leibowitz. 1994. Recombinant human IL-6 suppresses demyelination in a viral model of multiple sclerosis. J. Immunol. 153:3811-3821. [PubMed] [Google Scholar]
- 47.Roos, R. P., S. Firestone, R. Wollmann, D. Variakojis, and B. G. Arnason. 1982. The effect of short-term and chronic immunosuppression on Theiler's virus demyelination. J. Neuroimmunol. 2:223-234. [DOI] [PubMed] [Google Scholar]
- 48.Sato, S., S. L. Reiner, M. A. Jensen, and R. P. Roos. 1997. Central nervous system cytokine mRNA expression following Theiler's murine encephalomyelitis virus infection. J. Neuroimmunol. 76:213-223. [DOI] [PubMed] [Google Scholar]
- 49.Sedgwick, J. D., A. L. Ford, E. Foulcher, and R. Airriess. 1998. Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J. Immunol. 160:5320-5330. [PubMed] [Google Scholar]
- 50.Sedgwick, J. D., R. Mossner, S. Schwender, and V. ter Meulen. 1991. Major histocompatibility complex-expressing nonhematopoietic astroglial cells prime only CD8+ T lymphocytes: astroglial cells as perpetuators but not initiators of CD4+ T cell responses in the central nervous system. J. Exp. Med. 173:1235-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.So, E. Y., M. H. Kang, and B. S. Kim. 2006. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler's murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia 53:858-867. [DOI] [PubMed] [Google Scholar]
- 52.Theiler, M. 1937. Spontaneous encephalomyelitis of mice: a new virus disease. J. Exp. Med. 65:705-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Pesch, V., O. van Eyll, and T. Michiels. 2001. The leader protein of Theiler's virus inhibits immediate-early alpha/beta interferon production. J. Virol. 75:7811-7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wirenfeldt, M., A. A. Babcock, R. Ladeby, K. L. Lambertsen, F. Dagnaes-Hansen, R. G. Leslie, T. Owens, and B. Finsen. 2005. Reactive microgliosis engages distinct responses by microglial subpopulations after minor central nervous system injury. J. Neurosci. Res. 82:507-514. [DOI] [PubMed] [Google Scholar]
- 55.Yauch, R. L., K. Kerekes, K. Saujani, and B. S. Kim. 1995. Identification of a major T-cell epitope within VP3 amino acid residues 24 to 37 of Theiler's virus in demyelination-susceptible SJL/J mice. J. Virol. 69:7315-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yauch, R. L., and B. S. Kim. 1994. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler's virus is located within VP1(233-244). J. Immunol. 153:4508-4519. [PubMed] [Google Scholar]
- 57.Yauch, R. L., J. P. Palma, H. Yahikozawa, C. S. Koh, and B. S. Kim. 1998. Role of individual T-cell epitopes of Theiler's virus in the pathogenesis of demyelination correlates with the ability to induce a Th1 response. J. Virol. 72:6169-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]