Abstract
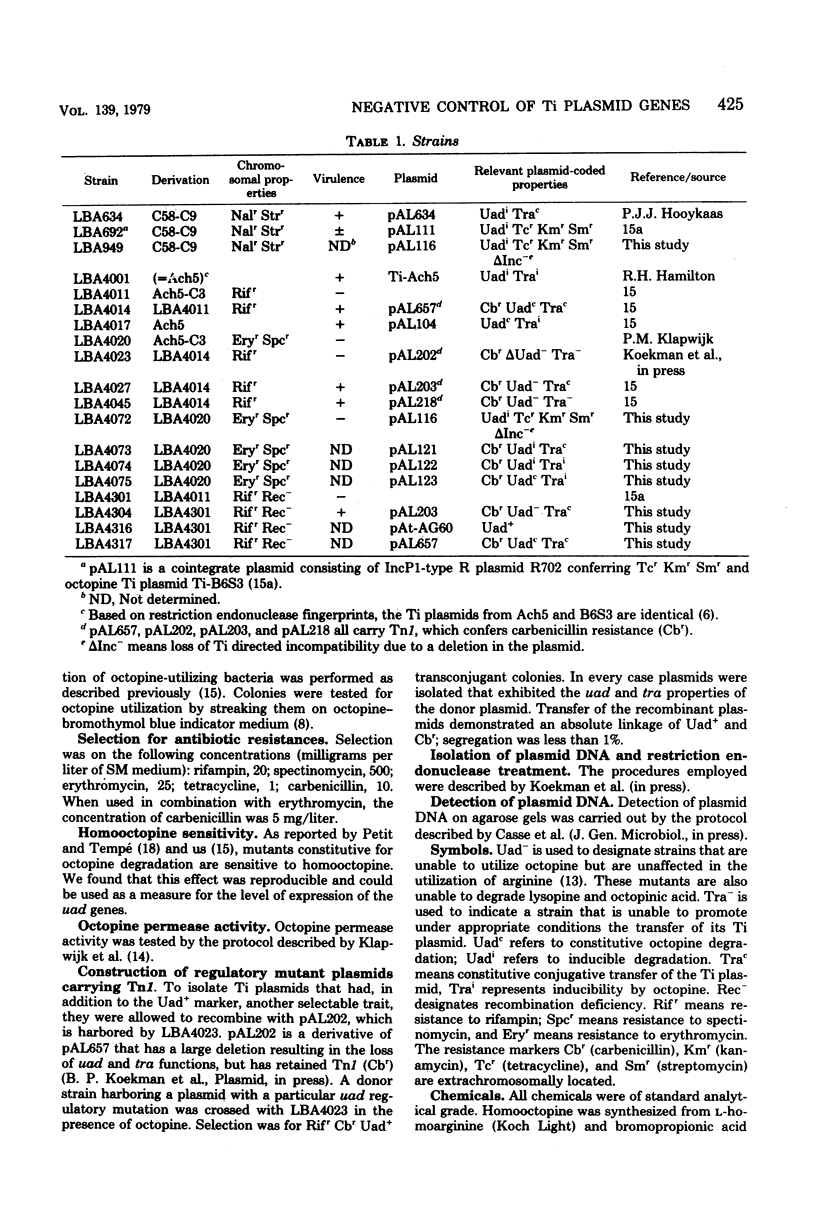
The regulatory system that controls the expression of the Ti plasmid-borne octopine degradation (uad) and transfer (tra) genes in Agrobacterium tumefaciens was studied. A deletion mutant derived from the cointegrate plasmid R702::Ti-B6S3 was isolated, which was compatible with a wild-type Ti plasmid and which had retained the uad genes. By means of this mutant plasmid pAL116, it was possible to make cells diploid for the uad genes. pAL116 was introduced into Rec- strains that contained different types of regulation mutants for the uad and tra genes. The repression pattern that was found in this complementation analysis indicated that the uad and tra operons are controlled by a common repressor system. Several results indicated that there may be additional transcriptional relations between both operons. The corresponding genes of the non-tumorigenic octopine plasmid pAt-AG60 appeared to be controlled by a repressor related to that of the octopine Ti plasmid.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Montoya A. L., Merlo D. J., Drummond M. H., Nutter R., Gordon M. P., Nester E. W. Restriction endonuclease mapping of a plasmid that confers oncogenicity upon Agrobacterium tumefaciens strain B6-806. Plasmid. 1978 Feb;1(2):254–269. doi: 10.1016/0147-619x(78)90043-4. [DOI] [PubMed] [Google Scholar]
- Drummond M. H., Chilton M. D. Tumor-inducing (Ti) plasmids of Agrobacterium share extensive regions of DNA homology. J Bacteriol. 1978 Dec;136(3):1178–1183. doi: 10.1128/jb.136.3.1178-1183.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The nature of the transfer inhibitor of several F-like plasmids. Mol Gen Genet. 1972;119(1):57–66. doi: 10.1007/BF00270444. [DOI] [PubMed] [Google Scholar]
- Genetello C., Van Larebeke N., Holsters M., De Picker A., Van Montagu M., Schell J. Ti plasmids of Agrobacterium as conjugative plasmids. Nature. 1977 Feb 10;265(5594):561–563. doi: 10.1038/265561a0. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jubier M. -F. Degradation of lysopine by an inducible membrane-bound oxidase in Agrobacterium tumefaciens. FEBS Lett. 1972 Dec 1;28(2):129–132. doi: 10.1016/0014-5793(72)80693-8. [DOI] [PubMed] [Google Scholar]
- Kerr A., Manigault P., Tempé J. Transfer of virulence in vivo and in vitro in Agrobacterium. Nature. 1977 Feb 10;265(5594):560–561. doi: 10.1038/265560a0. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., Hooykaas P. J., Kester H. C., Schilperoort R. A., RORSCH A. Isolation and characterization of Agrobacterium tumefaciens mutants affected in the utilization of octopine, octopinic acid and lysopine. J Gen Microbiol. 1976 Sep;96(1):155–163. doi: 10.1099/00221287-96-1-155. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., Scheulderman T., Schilperoort R. A. Coordinated regulation of octopine degradation and conjugative transfer of Ti plasmids in Agrobacterium tumefaciens: evidence for a common regulatory gene and separate operons. J Bacteriol. 1978 Nov;136(2):775–785. doi: 10.1128/jb.136.2.775-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk P. M., van Beelen P., Schilperoort R. A. Isolation of a recombination deficient Agrobacterium tumefaciens mutant. Mol Gen Genet. 1979 Jun 7;173(2):171–175. doi: 10.1007/BF00330307. [DOI] [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos C. G., Psallidas P. G. Characteristics of Greek Isolates of Agrobacterium tumefaciens (E. F. Smith & Townsend) Conn. J Appl Bacteriol. 1973 Jun;36(2):233–240. doi: 10.1111/j.1365-2672.1973.tb04096.x. [DOI] [PubMed] [Google Scholar]
- Sciaky D., Montoya A. L., Chilton M. D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]