Abstract
Simian immunodeficiency virus (SIV) infection of rhesus macaques (RMs) provides a reliable model to study the relationship between lentivirus replication, cellular immune responses, and CD4+ T-cell dynamics. Here we investigated, using SIVmac251-infected RMs of a Chinese genetic background (which experience a slower disease progression than Indian RMs), the dynamics of CD4+ CCR5+ T cells, as this subset of memory/activated CD4+ T cells is both a preferential target of virus replication and a marker of immune activation. As expected, we observed that the number of circulating CD4+ CCR5+ T cells decreases transiently at the time of peak viremia. However, at 60 days postinfection, i.e., when set-point viremia is established, the level of CD4+ CCR5+ T cells was increased compared to the baseline level. Interestingly, this increase correlated with faster disease progression, higher plasma viremia, and early loss of CD4+ T-cell function, as measured by CD4+ T-cell count, the fraction of memory CD4+ T cells, and the recall response to purified protein derivative. Taken together, these data show a key difference between the dynamics of the CD4+ CCR5+ T-cell pool (and its relationship with disease progression) in Chinese RMs and those described in previous reports for Indian SIVmac251-infected RMs. As the SIV-associated changes in the CD4+ CCR5+ T-cell pool reflect the opposing forces of SIV replication (which reduces this cellular pool) and immune activation (which increases it), our data suggest that in SIV-infected Chinese RMs the impact of immune activation is more prominent than that of virus replication in determining the size of the pool of CD4+ CCR5+ T cells in the periphery. As progression of HIV infection in humans also is associated with a relative expansion of the level of CD4+ CCR5+ T cells, we propose that SIV infection of Chinese RMs is a very valuable and important animal model for understanding the pathogenesis of human immunodeficiency virus infection.
Despite many years of intense study, the mechanisms of AIDS pathogenesis remain poorly understood. In particular, it is still unclear how the direct effect of virus replication, as opposed to the indirect effects of the host immune response (i.e., chronic immune activation), differentially contribute to determine the chronic and progressive loss of CD4+ T cells that is the best predictor of disease progression in human immunodeficiency virus (HIV)-infected individuals (5, 12).
The use of nonhuman primate models, particularly simian immunodeficiency virus (SIV)-infected Asian rhesus macaques (RMs), has allowed for the detailed and sequential investigation of the early events of SIV infection in terms of virus dynamics, immune responses to SIV, and changes in the pool of CD4+ T cells, particularly those expressing the main virus coreceptor, CCR5 (19, 23, 24, 27, 29, 36, 38, 47, 51-53, 55). These studies have evidenced that the primary acute phase of HIV and SIV infections is characterized by an early burst of virus replication that is reflected in an exponential increase in plasma viral load, lasting approximately 10 days and resulting in the dissemination and seeding of the virus in all peripheral lymphoid organs as well as mucosa-associated lymphoid tissues (3, 13, 18, 41, 46). The level of virus replication starts to decline soon after the initial peak and then reaches a plateau (set-point phase) that remains relatively constant for a long period of time, particularly in HIV-infected humans for whom the chronic phase of infection lasts for many years (21). The postpeak decline of virus replication likely is related to (i) the induction of the host immune response against the virus and (ii) the exhaustion of the pool of activated, CCR5-expressing CD4+ T cells that represents the optimal substrate for virus replication (23, 24, 36). Experiments aimed at identifying the relative contribution of these two mechanisms are complicated by the fact that the induction of T-cell responses to HIV/SIV results in the generation of more target cells for virus replication (as HIV and SIV preferentially replicate in activated CD4+ T cells [8]) and by the rapid development of viral escape from the host immune response (54).
The acute phase of HIV/SIV infection is characterized by a severe loss of CD4+ CCR5+ T cells, particularly in mucosal tissues (23, 51, 52); however, the meaning of this early depletion of mucosal CD4+ T cells still is unclear, as the same phenomenon has been observed during the acute stage of nonpathogenic SIV infections of sooty mangabeys (48) and African green monkeys (11, 33). Once the set-point phase is reached, the level of viral load predicts the rate of progression to AIDS (19, 21, 25, 27, 47, 55). The progressive decline of immune function is consistently associated with the loss of HIV/SIV-specific immune responses (4, 17), with HIV-infected nonprogressors being characterized by low viral load, preservation of the CD4+ T-cell pool, and broad T-cell responses to HIV (31, 34, 42, 43).
While SIVmac infection of RMs has proved an invaluable animal model for studies of AIDS pathogenesis, therapeutics, and vaccines, it is clear that substantial differences exist between the course of HIV infection in humans and that of SIV in RMs. Most notably, the average time to progress to AIDS is markedly shorter for SIVmac239- or SIVmac251-infected RMs than for untreated HIV-infected humans. For SIV-infected Indian RMs, the risk of progressing to AIDS is associated with a rapid and sustained depletion of circulating and mucosal CD4+ CCR5+ memory T cells (24). This observation represents a somewhat striking difference from HIV infection, as several studies reported that, in HIV-infected individuals, the proportion of CD4+ T cells expressing CCR5 greatly increases following HIV infection (7, 30), and that this increase in CD4+ CCR5+ T cells is a marker of disease progression (39). Collectively, these findings raise the concern that, beyond a series of obvious similarities, the SIVmac-infected RM model does not reproduce correctly the pathogenic events occurring during HIV infection, particularly with respect to the dynamics of the CD4+ CCR5+ T-cell subset. To this end, it should be noted that the two main driving forces behind the dynamics of CD4+ CCR5+ T cells during SIV infection are the direct virus infection (which tends to reduce the number of these cells) (23, 36, 51) and the immune activation that promotes the expression of CCR5 (and thus increases the number of CCR5+ T cells) (22, 29). As such, the differences in CD4+ CCR5+ T-cell dynamics observed between humans and RMs may reflect a different contribution of the two above-described mechanisms by which a retroviral infection affects this cellular subset.
We recently demonstrated that in SIV-infected Chinese RMs, for which the course of infection is slower than that of SIV-infected Indian RMs (thus making them more similar to HIV-infected individuals) (20, 37), the levels of immune activation and apoptosis are positively correlated with the rate of progression to AIDS (29, 53). In the current work, we tested the hypothesis that the dynamics of CCR5-expressing CD4+ T cells in SIV-infected Chinese RMs might be substantially different from those reported for SIV-infected RMs of an Indian genetic background (23, 24, 36, 51, 52). Based on a detailed study of the main virologic and immunologic parameters during primary SIVmac251 infection of a group of 10 Chinese RMs (divided into two groups: slow progressors [SPs] and normal progressors [NPs]), we determined that the dynamics of CD4+ CCR5+ T cells in SIV-infected Chinese RMs are more similar to those in HIV-infected humans. As such, we propose that SIV infection of Chinese RMs is an extremely useful and particularly relevant model to study AIDS pathogenesis and vaccines.
MATERIALS AND METHODS
Animals and virus infection.
RMs (Macaca mulatta) of Chinese origin were confirmed prior to infection as seronegative for simian T leukemia virus type-1, simian retrovirus type 1 (type D retrovirus), herpes B viruses, and SIVmac. All animals were housed in compliance with French regulations for animal care and usage (http://www.pasteur.fr/recherche/unites/animalerie/fichiers/Decret2001-486.pdf) and were inoculated intravenously with the pathogenic SIVmac251 strain (10 50% animal infectious doses). Animals were 8.50 ± 0.97 years old and had an initial CD4+ T-cell count in blood of 875 ± 518/mm3. While RMs of Indian origin often express the Manu-A*01 major histocompatibility complex class I allele that is associated with a better prognosis of SIV infection, this allele is not frequent in RMs of Chinese origin (1 out of 20 express this allele). Chinese RM genotyping is not readily available. The pathogenic SIVmac251 isolate initially was provided by R. Desrosiers and was titrated in Chinese rhesus macaques by intravenous inoculation. A 1-ml volume of stock virus contained 4 × 104 50% animal infectious doses (27).
Determination of viral load and quantitative assessment of productively infected cells.
RNA was extracted from the serum of SIV-infected macaques by using the Trireagent BD kit (Molecular Research Center Inc., Cincinnati, OH). Real-time quantitative reverse transcriptase-PCR was used to determine serum viral loads as previously described (28). We also quantified productively infected cells in lymph nodes by in situ hybridization using a 35S-labeled RNA probe derived from pBluescript carrying the SIVmac nef gene. The number of lymph-node-positive cells (spots), counted in the paracortical zone, is divided by the surface area of the entire lymph node section. Results then were expressed as the number of positive cells/2-mm2 section, as previously described (27). The mean count obtained for three slides is indicated.
Measurement of the frequency of SIV DNA+ in CD4+ T cells.
The frequency of SIV-infected cells was determined as previously reported (27) by limiting-dilution PCR of lymph-node-derived CD4+ T cells purified by sorting (FACS vantage; Becton Dickinson) from mononuclear cells with the positive selection of cells stained with a CD4-specific monoclonal antibody (MAb) (OKT4; Ortho Diagnostic). In selected experiments, cells also were costained for Ki67 or CCR5. Purified T cells were counted and diluted in series with a constant number of CEMx174 cells as the carrier. Cells were directly lysed with TPK buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 2.5 mM MgCl2, 0.5% Nonidet P-40, 0.5% Tween 20, 100 μg/ml of proteinase K). After incubation for 1 h at 56°C, proteinase K was inactivated at 95°C for 10 min. We subjected 20 limiting-dilution replicates to a nested PCR. SIV proviral DNA was amplified by nested PCR with SIVmac251-specific primers surrounding the nef region. After 35 cycles (95°C for 30 s, 60°C for 30 s, and 72°C for 1 min) with the first set of primers, Preco (5′-CAG AGG CTC TCT GCG ACC CTA C) and K3 (5′-GAC TGA ATA CAG AGC GAA ATG C), we amplified a fragment of 961 bp. We then reamplified 10 μl of product (30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min) with primers K1 (5′-TGG AAG ATG GAT CCT CGC AAT CC) and A2 (5′-GGA CTA ATT TCC ATA GCC AGC CA). Nested PCR products were subjected to electrophoresis in a 1.8% agarose gel. The proportion of infected T cells in the purified T-cell preparation was determined from Poisson statistics. The limiting-dilution PCR method was able to detect 1 infected cell in 10,000 uninfected cells (CEMx174), as demonstrated with SIV-1C cells (provided by F. Villinger) that contain a single SIVmac251 provirus per cell.
T-cell proliferation.
Standard proliferation assays were performed with freshly prepared cells as previously described (27). Peripheral blood mononuclear cells (PBMCs) were isolated from blood on a J-Prep density gradient (Techgen International, Voisins le Bretonneux, France) (1.077 g/ml). PBMCs (5 × 105 cells/well) were cultured at 37°C in a humidified atmosphere containing 5% CO2. Cells were incubated in the presence of a reverse transcriptase inhibitor (dideoxyinosine; 1 μM) for 6 days with a tuberculin antigen (purified protein derivative [PPD]; 30 μg/ml). [3H]thymidine (1 μCi) was added for the last overnight culture. Experiments were performed in triplicate. Results are expressed as a stimulation index. A stimulation index of 3 or more was considered significant. Cells also were activated with concanavalin A (5 μg/ml) and interleukin-2 (10 ng/ml).
Lymphocyte immunophenotyping by flow cytometry.
T cells were stained with the following fluorochrome-labeled monoclonal antibodies: anti-rhesus monkey CD3 conjugated with peridinin-chlorophyll protein (clone FN18; Biosource International), anti-human CD4 conjugated with phycoerythrin (PE) or conjugated with allophycocyanin (APC) (clone M-T477; BD Biosciences), anti-human CD45RA− fluorescein isothiocyanate (FITC) (clone 2H4; Beckman Coulter), and CD62L-PE (clone SK11; BD Biosciences). Chemokine receptor expression was quantified using anti-human CXCR4-PE (clone 12G5; BD Biosciences) and anti-human CCR5-PE (clone 3A9; BD Biosciences) antibodies. Antibodies were added to 100 μl of whole blood collected in EDTA or to 2 × 105 lymph node cells. Cells were incubated for 15 min at room temperature. Erythrocytes were lysed with 2 ml of diluted IOTest 3 lysis solution (Beckman Coulter). The cells then were washed once in PBA buffer (phosphate-buffered saline [PBS]-1% bovine serum albumin-10 mM NaN3) and resuspended in PBS containing 1% paraformaldehyde (PBS-PF) as previously described (28).
For Ki67 staining, 100 μl of whole blood collected in EDTA or 2 × 105 lymph node cells was incubated with CD3-PE and CD4-APC (clone MT477; BD Biosciences) for 15 min at room temperature as previously described (28). Erythrocytes then were lysed, fixed, and permeabilized with 2 ml of the Permeafix reagent (BD Biosciences) (20 min at room temperature). Cells were washed in PBA containing 0.05% saponin (PBA-Sap) and were incubated for 45 min with Ki67-FITC MAb (clone Ki67; DAKO). Cells were washed twice in PBA-Sap and resuspended in PBS-PF. An isotypic control was prepared in parallel for each sample, in which a mouse immunoglobulin G1-FITC antibody replaced Ki67-FITC and was used to set the gate for Ki67+ staining.
Immunohistochemical analyses.
Organs were cut into 4-μm sections on a cryostat, and the sections were stored at −80°C until use. Slides were immunostained using antibodies directed against CCR5 (BD Biosciences) and CD4 molecules (a mix of CD4 MAbs from BD Biosciences and Novocastra was used). For revelation, we used a kit from Vector Laboratories. The slides then were slightly counterstained with hematoxylin. These analyses were performed on four different slides, in a blind fashion, by two investigators.
Statistical analyses.
The Mann-Whitney U and paired Student's t tests were used to determine whether differences in means were significant if P < 0.05. Spearman's rank correlation, implemented from Statistica software, was used to evaluate correlations. Best-fit lines are shown. We checked for correlations between the rate of viral replication and all parameters analyzed, before and during primary SIV infection.
RESULTS
Viral and CD4+ T-cell dynamics during primary SIVmac251 infection of Chinese RMs.
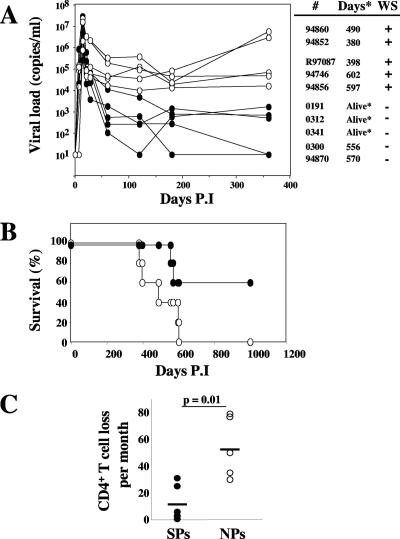
Infection of 10 Chinese RMs with the pathogenic SIVmac251 strain resulted in peak viremia (mean, 1.3 × 107 SIV RNA copies/ml; range, 2.6 × 105 to 3.6 × 107) by day 14 (Fig. 1A). As expected, the early peak in viremia was followed by a decline that resulted in levels of set-point viremia with a high degree of variability between individual animals (Fig. 1A). Based on their viral load levels at day 60, the 10 RMs of this cohort could be divided into a group of five animals with low virus replication (nos. 0191, 0300, 0341, 0312, and 94870; mean, 2.7 × 103 SIV+ RNA copies/ml; range, 102 to 104) and a group of five with high virus replication (nos. 94746, 94852, 94856, R97087, and 94860; mean, 1.2 × 105 SIV+ RNA copies/ml; range, 2 × 104 to 3.4 × 105). Similarly, at 6 months postinfection, viral loads remained less than 104 copies/ml in all RMs belonging to the first group but exceeded 104 copies/ml in all animals of the second group. By day 360 after infection, all five RMs with higher set-point viral loads had developed AIDS (Fig. 1B), while all five animals with lower set-point viral loads remained asymptomatic throughout the study (two of these RMs were later sacrificed as controls, while the remaining three were still alive after 4 years of infection). Based on this pattern of virus replication and survival, we classified the 10 RMs in this study into five NPs and five SPs. As expected from the dynamics of virus replication and the time to develop AIDS, the NPs displayed a more rapid decrease in CD4+ T-cell counts (53.2 ± 21.8 CD4+ T cells lost per month in NPs versus 14 ± 16.3 per month in SPs) (Fig. 1C). In all, these data confirm the well-known observation that the level of virus replication and rate of CD4+ T-cell decline are strong predictors of disease progression during pathogenic SIV infection of Chinese RMs (27-29, 53).
FIG. 1.
Viral dynamics during primary SIV infection. (A) Kinetic analysis of viremia in SIV-infected macaques and pathogenesis. Macaques were killed as indicated. Five developed a wasting syndrome (WS), whereas three were alive after 1,000 days of infection (*); each symbol represents one individual. SPs and NPs are identified by • and ○, respectively. Also shown are survival (in months) (B) and CD4+ T-cell loss per month (C) in SPs (•) and NPs (○). Data were compared using Student's t test. P.I, postinfection.
Early impairment of CD4+ T-cell function in SIVmac251-infected Chinese RMs.
It has been suggested that the early establishment of an effective CD4+ T-cell-mediated immune response to HIV is a determinant in slower disease progression (31, 42, 43). To better define the impact of SIVmac251 infection on the CD4+ T-cell pool in our group of Chinese RMs, we assessed longitudinally the relative proportion of naïve (CD62L+ CD45RA+), central memory (CD62L+ CD45RA−), effector memory (CD62L− CD45RA−), and terminally differentiated (CD62L− CD45RA+) CD4+ T cells (53). It should be noted that the expression of the main SIV coreceptor, CCR5, is almost exclusively restricted to non-naïve CD4+ T cells (23, 24, 36, 52).
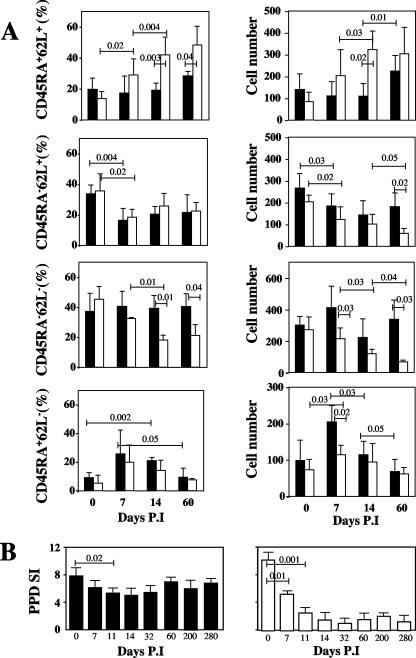
As shown in Fig. 2A, we observed that both the proportion and the absolute number of circulating naïve CD4+ T cells were higher for NPs than for SPs during the first 60 days of infection. In contrast, the relative proportion of central memory CD4+ T cells was similar for SPs and NPs, with a significant decline in both groups between days 0 and 7 (P = 0.02). Interestingly, the proportion of central memory CD4+ T cells stabilizes between days 7 and 60 for both SPs and NPs. In terms of absolute numbers, a more pronounced decline in the number of central memory CD4+ T cells was observed for NPs than SPs at day 60 (60 ± 21 cells and 182 ± 62, respectively; P = 0.02). Most importantly, our analysis of the dynamics of the main CD4+ T-cell subsets demonstrated a dramatic difference between NPs and SPs in the SIV-induced changes in both the proportion and absolute number of effector memory (CDR45RA− CD62L−) CD4+ T cells. Perhaps the most remarkable finding was the difference between the absolute number of effector memory CD4+ T cells in NPs and that in SPs at day 60 after infection (67 ± 12 and 339 ± 120, respectively; P = 0.03). Finally, we found only relatively minor changes in the proportion and absolute number of terminally differentiated (CD45RA+ CD62L) CD4+ T cells, both in terms of change from baseline and differences between SPs and NPs (Fig. 2A). Taken together, these data confirm the previously described phenomenon (36) that the loss of effector memory CD4+ T cells is a strong predictor of disease protection during SIV infection of RMs.
FIG. 2.
Loss of memory CD3+ CD4+ T cells during primary SIV infection. (A) Dynamics of T-cell subsets in SPs (▪) and NPs (□) during the acute phase. PBMCs were analyzed by flow cytometry on CD3+ CD4+ T cells. The percentages (left) and numbers (right) of CD45RA+ CD62L+, CD45RA− CD62L+, CD45RA− CD62L−, and CD45RA+ CD62L− T cells are shown. (B) Impaired T-cell proliferation against PPD during primary SIV infection. The proliferation of T cells specific for tuberculin (PPD; 30 μg/ml) was assessed from blood of SPs (▪) and NPs (□) before and during infection. Thymidine incorporation was determined on day 6, and results are expressed as a stimulation index (SI). Statistical significance was assessed using paired and unpaired Student's t tests. P.I, postinfection.
To further characterize the defect in memory CD4+ T-cell function induced by SIVmac251 in Chinese RMs, we next assessed the immunological response to recall antigens by measuring the tuberculin-specific in vitro T-cell proliferation (all animals were immunized with bacillus Calmette-Guerin 4 months before infection). As shown in Fig. 2B, we found that the lymphoproliferative response to PPD was markedly decreased within 14 days of infection (and remained low up to day 60) in the NPs, with only relatively minor changes observed in the SPs. These findings support the notion that memory CD4+ T-cell function is affected early after SIV infection and particularly in those RMs (i.e., the NPs) that will experience higher viral loads and faster disease progression.
Faster disease progression in SIVmac251-infected Chinese RMs is associated with a relative expansion of circulating CD4+ CCR5+ T cells.
Pathogenic SIV infection of Indian RMs is associated with a rapid decline of circulating as well as mucosal CD4+ CCR5+ T cells that is thought to be a key factor in the progression to simian AIDS (23, 24, 36, 51, 52). As shown in Fig. 3A for a representative animal, the vast majority of circulating CD4+ T cells in uninfected Chinese RMs expressed CXCR4, while a much smaller proportion of CD4+ T cells expressed CCR5 (29).
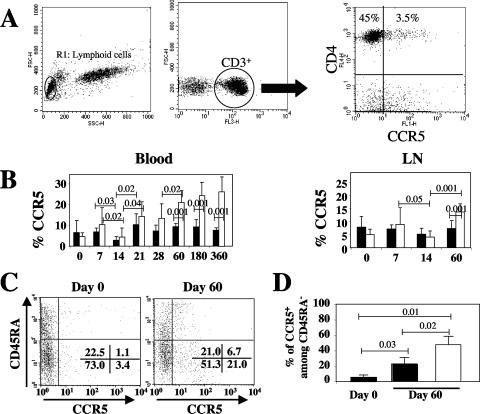
FIG. 3.
Dynamics of chemokine receptor expression on CD3+ CD4+ T cells during primary SIV infection. (A) Representative flow cytometry dot plots of CCR5 expression on CD4+ T cells from peripheral blood of uninfected macaques. Cells besides CD4+ T cells also are shown as a control. (B) The dynamics of CCR5 expression on CD3+ CD4+ T cells in peripheral blood and lymph nodes (LN) during primary SIV infection. SPs and NPs are identified as ▪ and □, respectively. (C) CCR5 expression versus CD45RA expression on CD4+ T cells. Representative dot plots of CD45RA with CCR5 on CD4+ T cells on days 0 and 60 are shown. The percentages are given in each quadrant. (D) The percentage of CCR5+ cells among CD45RA− CD4+ T cells at days 0 and 60 in SPs (▪) and NPs (□). Data were compared using a Student's t test.
We next assessed, in our group of SIV-infected Chinese RMs, the dynamics of CD4+ CCR5+ and CD4+ CXCR4+ T cells on circulating as well as lymph-node-derived CD4+ T cells. The proportion of CD4+ T cells in the blood and lymph nodes expressing CXCR4 showed only minimal changes during primary infection (data not shown). As expected, however, we observed that SIVmac251 infection induced significant changes in the relative proportion of CD4+ CCR5+ T cells in both peripheral blood and lymph nodes (Fig. 3B). Interestingly, we found a transient decline of CD4+ CCR5+ T cells at the peak of viral replication. In contrast to previous observations of SIV-infected Indian RMs (23, 24, 36), we found that by day 60 postinfection the relative proportion of CD4+ CCR5+ T cells in both peripheral blood and lymph nodes was significantly higher than the preinfection levels in NPs (20.9% ± 5.7% [P = 0.0004] and 13.6% ± 3.1% [P = 0.001] compared to levels from day 0 for blood and lymph nodes, respectively) (Fig. 3B). Similarly, during the chronic phase, the percentage of CD4+ CCR5+ T cells in peripheral blood remained higher in NPs than in SPs. To better characterize the phenotype of CD4+ CCR5+ T cells in our group of Chinese RMs, we next assessed changes of CCR5 expression on CD4+ CD45RA+ (i.e., largely naïve) and CD4+ CD45RA− (i.e., central and effector memory) T cells following SIVmac251 infection. As shown in Fig. 3C for a representative animal, we found that the majority of CD4+ CCR5+ T cells belong to the CD45RA− population both at baseline and at day 60 postinfection. Importantly, the frequency of CCR5-expressing central/effector memory (i.e., CD45RA−) CD4+ T cells at day 60 postinfection was significantly higher in NPs than in SPs (Fig. 3D).
Collectively, these data indicate that the fraction of CD4+ CCR5+ T cells and, more specifically, the fraction of CD45RA− CCR5+ central/effector memory CD4+ T cells in peripheral blood are significantly higher in SIVmac251-infected Chinese RMs that exhibit faster disease progression. As such, our current findings identify a specific feature of the dynamics of CD4+ CCR5+ T cells in Chinese RMs, in which progression is closely associated with a CD4+ CCR5+ T-cell increase in the periphery. This is in contrast to the observation that, in SIV-infected Indian RMs, disease progression is associated with the depletion of circulating CD4+ CCR5+ T cells (23, 24, 36, 51, 52).
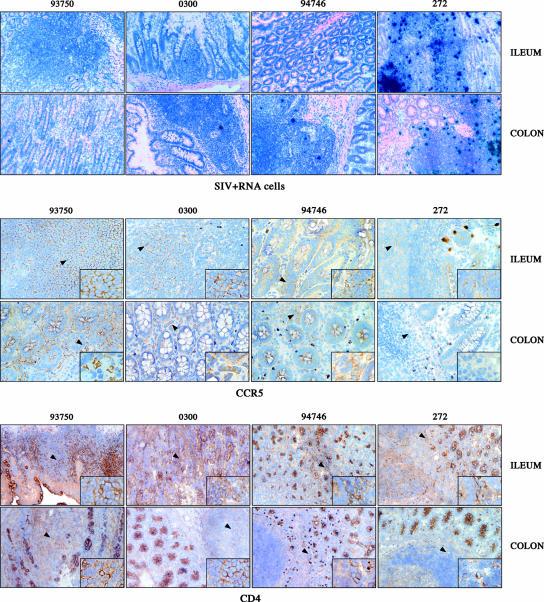
The early and persistent depletion of mucosal CD4+ T cells currently is considered a major determining factor in the pathogenesis of SIV infection in Indian RMs (23, 36, 49). To define the impact of SIV infection on mucosal CD4+ T cells in Chinese RMs, we examined the expression of CD4 and CCR5 as well as the level of productively infected cells (SIV RNA+ cells) in the ileum and colon of SIV-infected Chinese RMs at the time of death (Fig. 4). This analysis includes an RM (no. 272) with rapid progression towards AIDS that was sacrificed after 6 months due to a wasting syndrome. As shown in Fig. 4, we found that SIV RNA+ cells are present at high levels within the ileum and the colon of macaque 272, concomitant with a marked depletion of CD4+ and CCR5+ cells in the lamina propria. In contrast, fewer SIV RNA+ cells were detected in the two other studied RMs. Interestingly, the depletion of CD4+ T cells from the lamina propria of SIV-infected Chinese RMs was associated with a relative preservation of the cells in the organized lymphoid aggregates of the inductive mucosal immune system (Peyer's patches and lymphoid follicles). In SP Chinese RMs, the level of CCR5+ cells in the lamina propria was lower than that in uninfected animals but was higher than that in NPs. In all, these results suggest a better recovery of CD4+ and CCR5+ cells in SP SIV-infected Chinese RMs than in NPs.
FIG. 4.
Immunohistochemical analyses of CD4 (bottom) and CCR5 (middle) expression in ileum and colon (magnification, ×100) of uninfected (no. 93750) and SIV-infected macaques displaying distinct rates of progression towards AIDS (SP, no. 0300; NP, no. 94746; rapid progressor, no. 272). SIV RNA+ cells also were detected in the same animals (top).
Dynamics of cycling CD4+ T cells during primary SIVmac251 infection of Chinese RMs.
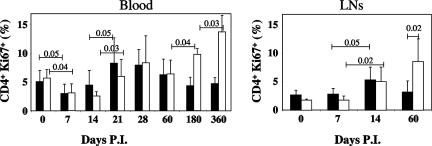
As we found that SIVmac251 infection of Chinese RMs is associated with significant changes in the proportion of CD4+ CCR5+ T cells, we reasoned that these changes might reflect the prevailing level of immune activation. To directly investigate the changes in T-cell activation occurring in our group of SIVmac251-infected Chinese RMs, we longitudinally evaluated the fraction of cycling CD4+ T cells by monitoring the expression of Ki67, a nuclear antigen expressed in the G1, S, G2, and M phases, but not in the G0 phase, of the cell cycle (28).
As shown in Fig. 5, we found that the percentage of circulating CD4+ Ki67+ T cells decreased slightly but transiently during the acute phase (3.05% ± 1.51% on day 7 and 3.51% ± 2.04% on day 14 versus 5.38% ± 1.64% before infection; P = 0.007 and P = 0.015, respectively). This was followed by a rebound on days 21 and 28, achieving up to twofold higher levels than those observed before infection. Interestingly, no statistically significant differences were observed between NPs and SPs in terms of Ki67 expression in the blood (Fig. 5). In the lymph nodes, the percentage of CD4+ Ki67+ T cells increased by day 14 (4.67% ± 2.48% versus 2.07% ± 0.93 before infection; P = 0.002) in both NPs and SPs (Fig. 5). By the end of the acute phase, i.e., at day 60, the increase in the fraction of CD4+ Ki67+ T cells was significantly higher in NPs than in SPs (8.54% ± 4.0% and 3.19% ± 1.97%, respectively; P = 0.02) (Fig. 5). Consistent with previous findings (28), this increase was higher in peripheral blood of chronically SIV-infected Chinese RMs that progress more rapidly to AIDS. Collectively, these data suggest that SIV infection of Chinese RMs is associated with a significant increase in the level of T-cell activation in both peripheral blood and lymph nodes, thus confirming the results of previous studies indicating an increase in CD4+ T-cell turnover during pathogenic SIV infection (26, 28, 44).
FIG. 5.
Dynamics of cycling CD4+ T cells during primary SIV infection. The percentage of CD4+ T cells expressing Ki67 was assessed by flow cytometry from blood or fresh lymph node (LN) cells of SPs (▪) or NPs (□). Data were compared using Student's t test. P.I., postinfection.
The level of SIV replication during the set-point phase correlates directly with the fraction of CD4+ CCR5+ T cells.
In the next set of experiments, we sought to define the relationship between virus replication and dynamics of the CD4+ CCR5+ T-cell subset in our group of SIVmac251-infected Chinese RMs. To generate a more complete set of data regarding SIV replication in these animals, we first assessed proviral DNA in lymph-node-derived CD4+ T cells at the time of peak of virus replication and then at day 60 postinfection. Consistent with our previous observations (27), we found that 2% ± 1.5% of sorted lymph-node-derived CD4+ T cells from SIV-infected macaques contained proviral DNA, with the frequency of SIV+ DNA peaking at day 14 after infection and then declining thereafter (data not shown). The frequency of proviral DNA in sorted CD4+ CCR5+ T cells was 0.8% ± 1.2%.
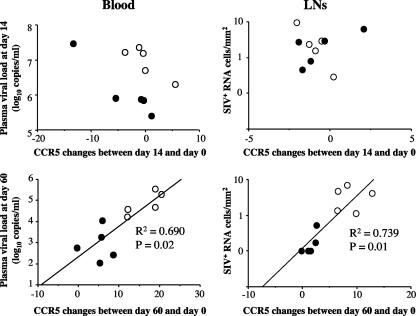
As shown in Fig. 6, we next investigated the relationship between SIV replication and changes in the level of circulating CD4+ CCR5+ T cells. We first observed that the level of virus replication during the acute phase of infection (i.e., day 14) was not correlated with changes in the level of CD4+ CCR5+ T cells, suggesting that direct virus killing (lysis of infected cells [35]) alone is unlikely to be the main driving force that determines the size of the circulating CD4+ CCR5+ T-cell pool during acute SIVmac251 infection of Chinese RMs. Interestingly, a significant direct correlation emerged by day 60 in both blood and lymph nodes between virus replication and the level of CD4+ CCR5+ T cells (R2 = 0.69 with P = 0.02 and R2 = 0.739 with P = 0.01 in blood and lymph nodes, respectively) (Fig. 6).
FIG. 6.
Correlation between chemokine receptor expression and the extent of viral replication. We plotted changes in CCR5 between days 14 and 0 and between days 60 and 0 of SPs (•) and NPs (○) against plasma viral loads (log10 copies/milliliter) and productively infected cells (SIV RNA+ cells/2 mm2) on days 14 and 60. Spearman's rank correlation coefficients were calculated to evaluate correlations.
Importantly, these data indicate that SIVmac251-infected RMs that show signs of rapid disease progression (assessed as high levels of virus replication) experience an increase in the level of circulating and lymph-node-derived CD4+ CCR5+ T cells. These findings highlight an important difference between Chinese and Indian RMs in how SIV infection affects the pool of CD4+ CCR5+ T cells (23, 24, 36).
DISCUSSION
Experimental SIV infection of RMs usually is conducted in animals of Indian origin, and for many years this has been the most widely used model to study AIDS pathogenesis and vaccines in vivo. However, there are a number of significant differences between this infection and HIV infection of humans. Among these differences, the most important are (i) a much shorter average time for progression to AIDS in SIVmac-infected RMs than in HIV-infected humans and (ii) the fact that peripheral blood CD4+ T-cell counts are not good predictors of progression to AIDS (20). Instead, in SIV-infected Indian RMs, the decline of memory CD4+ CCR5+ T cells appears to be the best predictor of disease progression (23). It should be noted that the latter finding is in obvious contrast to the observation that the proportion of CD4+ CCR5+ T cells is increased in the blood in HIV-infected individuals and that, in fact, the magnitude of this increase of CD4+ CCR5+ T cells is a marker of disease progression (7, 30, 39). As such, these differences highlight the limitations of the SIVmac model of infection of Indian RMs as truly representative of the immunopathogenic events taking place during HIV infection of humans.
In this report, we focused our attention on SIVmac251 infection of RMs of Chinese origin. In previous studies, it was shown that Chinese RMs experience a slower disease progression than Indian RMs (20, 37). Intriguingly, we have shown previously (1, 28, 29, 53) that, in SIV-infected Chinese RMs, the levels of immune activation and apoptosis are clear markers of disease progression, thus identifying a striking similarity to disease progression for HIV-infected humans, in whom the level of immune activation and death are better predictors of progression to AIDS than is viral load (6, 9, 10, 16). In the current study, we investigated for the first time the SIV-associated dynamics of CD4+ CCR5+ T cells in a group of 10 SIVmac251-infected Chinese RMs that, based on their outcome of infection, were divided into two groups of five NPs and five SPs. As expected, we observed a transient decline of circulating CD4+ CCR5+ T cells at the time of peak viremia that then was followed by a relative increase in the fraction of CD4+ CCR5+ T cells during the chronic phase. The most interesting part of this study, at least in our opinion, is that, in this group of SIVmac251-infected Chinese RMs, this relative expansion of the CD4+ CCR5+ T cells was correlated with all markers of active disease progression, including (i) higher virus replication, measured both as plasma viremia and cell-associated SIV in blood- or lymph-node-derived CD4+ T cells; (ii) overall loss of CD4+ T-cell function, measured by lower CD4+ T-cell counts, a relative decline of memory cells, and the failure to mount a recall response to PPD; and (iii) time to develop AIDS and overall survival. Taken together, these data identify a remarkable difference between Indian and Chinese RMs after SIVmac infection, with disease progression being associated with the rapid and progressive loss of CD4+ CCR5+ T cells in Indian RMs and, conversely, with a relative expansion of the levels of these cells during the chronic phase in SIV-infected Chinese RMs.
To fully appreciate this striking difference between Indian and Chinese RMs with respect to how SIV infection affects the dynamics of CD4+ CCR5+ T cells, it should be remembered that there are two main driving forces determining the size of CD4+ CCR5+ T-cell pools during SIV infection. The first force is the direct impact of virus replication, which targets memory/activated CD4+ CCR5+ T cells as they express the main coreceptor for SIV entry and display an activated metabolic state that supports virus production. The net effect of virus replication on the size of the CD4+ CCR5+ T-cell pool is towards a rapid depletion of these cells, as elegantly shown in a series of seminal studies (23, 36, 51). The second force that determines the size of the CD4+ CCR5+ T-cell pool during a retroviral infection is the prevailing level of immune activation, which promotes the expression of CCR5 on CD4+ T cells that previously had low levels of CCR5 (2, 22). As such, the net effect of the immune activation is to induce a relative expansion of the pool of CD4+ CCR5+ T cells, at least when these cells are enumerated as a fraction of the total CD4+ T-cell pool. Based on these considerations, it appears that the dynamics of the CD4+ CCR5+ T-cell subsets are profoundly different during SIVmac infection of Indian and Chinese RMs in the periphery. In Indian RMs, the drastic decline of the pool of CD4+ CCR5+ T cells is a key determinant of progression to AIDS during both acute and chronic infection, thus strongly suggesting that, in this animal model, the direct effect (i.e., death of productively infected cells) of virus replication (23) is the main determinant of the immune dysfunction that underlies the progression to AIDS and death. In contrast, Chinese RMs, which progress more slowly to AIDS, experience a relative expansion of the CD4+ CCR5+ T-cell pool, the extent of which clearly is associated with all markers of disease progression, thus indicating that the effect of immune activation (rather than the direct consequences of cell lysis mediated by productive infection [35]) is a key determinant in the immunological changes associated with progression to AIDS in these animals. This conclusion is consistent with the observation that, in SIV-infected Chinese RMs, the frequency of SIV DNA+ CD4+ CCR5+ T cells is relatively low throughout the study period. In addition, our data support the model proposed by Li et al. (18), in which the majority of SIV-associated CD4+ T-cell depletion is induced by indirect mechanisms such as the CD95-dependent apoptosis of uninfected cells. As the relative size of the pool of circulating CD4+ CCR5+ T cells (measured as a percentage of the total number of CD4+ T cells) also increases during HIV infection coincident with disease progression (7, 30, 39, 40), our current data identify an important similarity in the immunopathogenic events underlying disease progression in SIVmac-infected Chinese RMs and HIV-infected humans. At least in this regard, it is clear that SIVmac infection of Chinese RMs is a better model than SIVmac infection of Indian RMs.
When we examined mucosal tissues in our cohort of SIV-infected Chinese RMs, we found that CD4+ and CCR5+ cells are detectable both in lymphoid aggregates (i.e., Peyer's patches and isolates lymphoid follicles) and, to a lesser extent, in the lamina propria as well, with SPs showing a higher frequency of CD4+ and CCR5+ cells than NPs. This finding is somewhat in contrast with the previous observation that, in SIV-infected Indian RMs, progression to AIDS is closely associated with a rapid, dramatic, and persistent depletion of CD4+ CCR5+ T cells from mucosal sites (23, 51). In addition, our data are in agreement with a previous observation that in SIV-infected Chinese RMs, a progressive reconstitution of the pool of CD4+ CCR5+ T cells is observed in animals that do not progress to AIDS (20). In all, our comparative analysis of the pools of CD4+ CCR5+ T cells in blood, lymph nodes, and mucosal tissues indicates that, in SIV-infected Chinese RMs, the observed changes in this cell subset reflect a complex dynamic influenced by various factors, such as virus replication, immune activation, redistribution among anatomic compartments, and de novo production of CD4+ T cells. Ultimately, however, the observation of a relative expansion of memory CD4+ CCR5+ T cells in SIV-infected Chinese RMs indicates that the impact of immune activation and increased T-cell turnover outweighs the effect of the virus in determining the relative size of this cell subset. This conclusion is not entirely unexpected if one considers that virus replication in SIV-infected Chinese RMs is at a significantly lower level than that in Indian RMs.
The striking difference in the SIV-induced dynamics of CD4+ CCR5+ T cells between Indian and Chinese RMs likely is caused by genetic differences between these two types of animals. Genetic factors that may determine this divergent course of disease include different regulation of CCR5 expression, the presence of cellular molecules that restrict the level of SIV replication, the type and magnitude of SIV-specific immune responses, and the mechanisms by which the CD4+ T-cell homeostasis is maintained at the level of bone marrow, thymus, and lymph nodes. Interestingly, Picker et al. (36) have reported a level of CCR5-expressing CD4+ T cells of 21.8% ± 7.7% in healthy RMs of Indian origin, which is higher than the percentage of CCR5-expressing CD4+ T cells that we found in uninfected Chinese RMs (6.7% ± 4.6%). This higher level of baseline CCR5 expression in CD4+ T cells of Indian RMs might be a key reason underlying the greater susceptibility to SIV infection of these animals. This possibility also is consistent with the recent finding that CCR5 expression typically is low on CD4+ T cells of natural SIV hosts, such as sooty mangabeys and African green monkeys, for which the infection usually is nonpathogenic (32). As the level of SIV replication actually is fairly high (average set point of >105 copies/ml) in natural SIV hosts, we and others have proposed that the low levels of CCR5 expression of CD4+ T cells of these animals protect from disease progression not only by decreasing the number of available target cells but also by reducing the level of immune activation (15, 32).
The transient depletion of peripheral CD4+ CCR5+ T cells observed in our group of Chinese RMs at the time of peak viremia could be explained in several ways. A first possibility is that primary SIV infection induces chemokine receptor internalization, thus leading to an apparent decrease in the number of CD4+ T cells expressing CCR5. However, intracellular staining of permeabilized cells showed no significant differences in the amount of CCR5 expressed on CD4+ T cells between days 0, 7, and 14 (data not shown). A second possibility is that the acute SIV infection phase is associated with changes in the homing properties of CD4+ CCR5+, resulting in more rapid migration from blood and lymph nodes to effector mucosal sites. While we cannot formally rule out these two possibilities, we favor the hypothesis that the early but transient decline of the fraction of circulating CD4+ CCR5+ T cells simply reflects the direct effect of virus replication at a time when the immune activation associated with chronic infection has not yet developed.
Intriguingly, our study also shows that, in SIV-infected Chinese RMs, the selective loss of both central and effector memory CD4+ T cells (i.e., CD45RA− CD62L+ and CD45RA− CD62L−, respectively) is not associated with a concomitant selective depletion of CD4+ CCR5+ T cells. As such, our study indicates that, in this model of infection, the loss of memory CD4+ cells cannot simply be a consequence of the virus-mediated depletion of CD4+ CCR5+ T cells. If the SIV-mediated depletion of CD4+ CCR5+ T cells alone cannot provide a satisfactory explanation for the depletion of central/effector memory CD4+ T cells, the next question is the following: What are the cellular and molecular mechanisms linking the SIV-associated immune activation to the progressive attrition of the memory CD4+ T-cell pool? These mechanisms may include the collateral cell damage induced by the interaction of the viral envelope glycoprotein (gp120) with the CD4 molecule in uninfected cells (15) involving alternative coreceptors (50, 53) as well as the draining of the pool of memory CD4+ T cells through continuous recruitment of these cells into a pool of short-lived effector cells (14, 45).
In summary, the current findings indicate that, in SIVmac251-infected Chinese RMs, the set-point phase of infection is associated with a relative expansion of the circulating pool of CD4+ CCR5+ T cells, the extent of which correlates with all markers of SIV disease progression. These findings identify a key difference in the immunopathogenesis of infection between Chinese and Indian RMs, for which disease is closely associated with rapid and progressive depletion of CD4+ CCR5+ T cells (23, 24, 36). As progression of HIV infection in humans also is associated with a relative expansion of CD4+ CCR5+ T cells, we propose that SIV infection of Chinese RMs is a better model than the commonly used SIV infection of Indian RMs in reproducing the immunopathogenic events occurring during HIV infection of humans.
Acknowledgments
We thank R. Ho Tsong Fang for clinical care and P. Corbeau for helpful discussions. We also thank R. P. Sekaly for reviewing the manuscript. We also acknowledge J. M. Panaud (Institut Pasteur) for pictures.
This work was funded by grants from the Agence Nationale de Recherche sur le Sida (ANRS) and Fondation de France and Sidaction to J.E. F.P. was supported by a postdoctoral fellowship from Sidaction, and L.V. was supported by a doctoral fellowship from ANRS.
Footnotes
Published ahead of print on 26 September 2007.
This work is dedicated to the memory of B. Hurtel.
REFERENCES
- 1.Arnoult, D., F. Petit, J. D. Lelievie, D. Lecossier, A. Hance, V. Monceaux, R. Ho Tsong Fang, B. Huntrel, J. C. Ameisen, and J. Estaquier. 2003. Caspase-dependent and -independent T-cell death pathways in pathogenic simian immunodeficiency virus infection: relationship to disease progression. Cell Death Differ. 10:1240-1252. [DOI] [PubMed] [Google Scholar]
- 2.Bleul, C. C., L. Wu, J. A. Hoxie, T. A. Springer, and C. R. Mackay. 1997. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA 94:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarti, L., M. C. Cumont, L. Montagnier, and B. Hurtrel. 1994. Variable course of primary simian immunodeficiency virus infection in lymph nodes: relation to disease progression. J. Virol. 68:6634-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clerici, M., N. I. Stocks, R. A. Zajac, R. N. Boswell, D. R. Lucey, C. S. Via, and G. M. Shearer. 1989. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J. Clin. Investig. 84:1892-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, O. J., A. Kinter, and A. S. Fauci. 1997. Host factors in the pathogenesis of HIV disease. Immunol. Rev. 159:31-48. [DOI] [PubMed] [Google Scholar]
- 6.Deeks, S. G., C. M. Kitchen, L. Liu, H. Guo, R. Gascon, A. B. Narvaez, P. Hunt, J. N. Martin, J. O. Kahn, J. Levy, M. S. McGrath, and F. M. Hecht. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942-947. [DOI] [PubMed] [Google Scholar]
- 7.de Roda Husman, A. M., H. Blaak, M. Brouwer, and H. Schuitemaker. 1999. CC chemokine receptor 5 cell-surface expression in relation to CC chemokine receptor 5 genotype and the clinical course of HIV-1 infection. J. Immunol. 163:4597-4603. [PubMed] [Google Scholar]
- 8.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 9.Estaquier, J., T. Idziorek, F. de Bels, F. Barre-Sinoussi, B. Hurtrel, A. M. Aubertin, A. Venet, M. Mehtali, E. Muchmore, P. Michel, et al. 1994. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. USA 91:9431-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estaquier, J., T. Idziorek, W. Zou, D. Emilie, C. M. Farber, J. M. Bourez, and J. C. Ameisen. 1995. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J. Exp. Med. 182:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon, S. N., N. R. Klatt, S. E. Bosinger, J. M. Brenchley, J. M. Milush, J. C. Engram, R. M. Dunham, M. Paiardini, S. Klucking, A. Danesh, E. A. Strobert, C. Apetrei, I. V. Pandrea, D. Kelvin, D. C. Douek, S. I. Staprans, D. L. Sodora, and G. Silvestri. 2007. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 179:3026-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haase, A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625-656. [DOI] [PubMed] [Google Scholar]
- 13.Heise, C., P. Vogel, C. J. Miller, C. H. Halsted, and S. Dandekar. 1993. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am. J. Pathol. 142:1759-1771. [PMC free article] [PubMed] [Google Scholar]
- 14.Hellerstein, M. K., R. A. Hoh, M. B. Hanley, D. Cesar, D. Lee, R. A. Neese, and J. M. McCune. 2003. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J. Clin. Investig. 112:956-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurtrel, B., F. Petit, D. Arnoult, M. Muller-Trutwin, G. Silvestri, and J. Estaquier. 2005. Apoptosis in SIV infection. Cell Death Differ. 12(Suppl. 1):979-990. [DOI] [PubMed] [Google Scholar]
- 16.Katsikis, P. D., E. S. Wunderlich, C. A. Smith, and L. A. Herzenberg. 1995. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J. Exp. Med. 181:2029-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane, H. C., J. M. Depper, W. C. Greene, G. Whalen, T. A. Waldmann, and A. S. Fauci. 1985. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N. Engl. J. Med. 313:79-84. [DOI] [PubMed] [Google Scholar]
- 18.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 19.Lifson, J. D., M. A. Nowak, S. Goldstein, J. L. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. L. Lloyd, J. Williams, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 71:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling, B., R. S. Veazey, A. Luckay, C. Penedo, K. Xu, J. D. Lifson, and P. A. Marx. 2002. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS 16:1489-1496. [DOI] [PubMed] [Google Scholar]
- 21.Little, S. J., A. R. McLean, C. A. Spina, D. D. Richman, and D. V. Havlir. 1999. Viral dynamics of acute HIV-1 infection. J. Exp. Med. 190:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loetscher, P., M. Seitz, M. Baggiolini, and B. Moser. 1996. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J. Exp. Med. 184:569-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 24.Mattapallil, J. J., N. L. Letvin, and M. Roederer. 2004. T-cell dynamics during acute SIV infection. AIDS 18:13-23. [DOI] [PubMed] [Google Scholar]
- 25.Mellors, J. W., L. A. Kingsley, C. R. Rinaldo, Jr., J. A. Todd, B. S. Hoo, R. P. Kokka, and P. Gupta. 1995. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann. Intern. Med. 122:573-579. [DOI] [PubMed] [Google Scholar]
- 26.Mohri, H., S. Bonhoeffer, S. Monard, A. S. Perelson, and D. D. Ho. 1998. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science 279:1223-1227. [DOI] [PubMed] [Google Scholar]
- 27.Monceaux, V., J. Estaquier, M. Fevrier, M. C. Cumont, Y. Riviere, A. M. Aubertin, J. C. Ameisen, and B. Hurtrel. 2003. Extensive apoptosis in lymphoid organs during primary SIV infection predicts rapid progression towards AIDS. AIDS 17:1585-1596. [DOI] [PubMed] [Google Scholar]
- 28.Monceaux, V., R. H. Fang, M. C. Cumont, B. Hurtrel, and J. Estaquier. 2003. Distinct Cycling CD4+ and CD8+ T-cell profiles during the asymptomatic phase of simian immunodeficiency virus SIVmac251 infection in rhesus macaques. J. Virol. 77:10047-10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monceaux, V., L. Viollet, F. Petit, R. H. Fang, M. C. Cumont, J. Zaunders, B. Hurtrel, and J. Estaquier. 2005. CD8+ T cell dynamics during primary simian immunodeficiency virus infection in macaques: relationship of effector cell differentiation with the extent of viral replication. J. Immunol. 174:6898-6908. [DOI] [PubMed] [Google Scholar]
- 30.Ostrowski, M. A., S. J. Justement, A. Catanzaro, C. A. Hallahan, L. A. Ehler, S. B. Mizell, P. N. Kumar, J. A. Mican, T. W. Chun, and A. S. Fauci. 1998. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J. Immunol. 161:3195-3201. [PubMed] [Google Scholar]
- 31.Oxenius, A., D. A. Price, P. J. Easterbrook, C. A. O'Callaghan, A. D. Kelleher, J. A. Whelan, G. Sontag, A. K. Sewell, and R. E. Phillips. 2000. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA 97:3382-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandrea, I., C. Apetrei, S. N. Gordon, J. Barbercheck, J. Dufour, R. Bohm, B. S. Sumpter, P. Roques, P. A. Marx, V. M. Hirsch, A. Kaur, A. A. Lackner, R. S. Veazey, and G. Silvestri. 2007. Paucity of CD4+CCR5+ T-cells is a typical feature of natural SIV hosts. Blood 109:1069-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandrea, I. V., R. Gautam, R. M. Ribeiro, J. M. Brenchley, I. F. Butler, M. Pattison, T. Rasmussen, P. A. Marx, G. Silvestri, A. A. Lackner, A. S. Perelson, D. C. Douek, R. S. Veazey, and C. Apetrei. 2007. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J. Immunol. 179:3035-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantaleo, G., J. F. Demarest, T. Schacker, M. Vaccarezza, O. J. Cohen, M. Daucher, C. Graziosi, S. S. Schnittman, T. C. Quinn, G. M. Shaw, L. Perrin, G. Tambussi, A. Lazzarin, R. P. Sekaly, H. Soudeyns, L. Corey, and A. S. Fauci. 1997. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc. Natl. Acad. Sci. USA 94:254-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petit, F., D. Arnoult, J. D. Lelievre, L. M. Parseval, A. J. Hance, P. Schneider, J. Corbeil, J. C. Ameisen, and J. Estaquier. 2002. Productive HIV-1 infection of primary CD4+ T cells induces mitochondrial membrane permeabilization leading to a caspase-independent cell death. J. Biol. Chem. 277:1477-1487. [DOI] [PubMed] [Google Scholar]
- 36.Picker, L. J., S. I. Hagen, R. Lum, E. F. Reed-Inderbitzin, L. M. Daly, A. W. Sylwester, J. M. Walker, D. C. Siess, M. Piatak, Jr., C. Wang, D. B. Allison, V. C. Maino, J. D. Lifson, T. Kodama, and M. K. Axthelm. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200:1299-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reimann, K. A., R. A. Parker, M. S. Seaman, K. Beaudry, M. Beddall, L. Peterson, K. C. Williams, R. S. Veazey, D. C. Montefiori, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Pathogenicity of simian-human immunodeficiency virus SHIV-89.6P and SIVmac is attenuated in cynomolgus macaques and associated with early T-lymphocyte responses. J. Virol. 79:8878-8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimann, K. A., K. Tenner-Racz, P. Racz, D. C. Montefiori, Y. Yasutomi, W. Lin, B. J. Ransil, and N. L. Letvin. 1994. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Virol. 68:2362-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynes, J., P. Portales, M. Segondy, V. Baillat, P. Andre, O. Avinens, M. C. Picot, J. Clot, J. F. Eliaou, and P. Corbeau. 2001. CD4 T cell surface CCR5 density as a host factor in HIV-1 disease progression. AIDS 15:1627-1634. [DOI] [PubMed] [Google Scholar]
- 40.Reynes, J., P. Portales, M. Segondy, V. Baillat, P. Andre, B. Reant, O. Avinens, G. Couderc, M. Benkirane, J. Clot, J. F. Eliaou, and P. Corbeau. 2000. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J. Infect. Dis. 181:927-932. [DOI] [PubMed] [Google Scholar]
- 41.Ringler, D. J., M. S. Wyand, D. G. Walsh, J. J. MacKey, L. V. Chalifoux, M. Popovic, A. A. Minassian, P. K. Sehgal, M. D. Daniel, R. C. Desrosiers, et al. 1989. Cellular localization of simian immunodeficiency virus in lymphoid tissues. I. Immunohistochemistry and electron microscopy. Am. J. Pathol. 134:373-383. [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 44.Rosenzweig, M., M. A. DeMaria, D. M. Harper, S. Friedrich, R. K. Jain, and R. P. Johnson. 1998. Increased rates of CD4+ and CD8+ T lymphocyte turnover in simian immunodeficiency virus-infected macaques. Proc. Natl. Acad. Sci. USA 95:6388-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silvestri, G., and M. B. Feinberg. 2003. Turnover of lymphocytes and conceptual paradigms in HIV infection. J. Clin. Investig. 112:821-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sopper, S., D. Nierwetberg, A. Halbach, U. Sauer, C. Scheller, C. Stahl-Hennig, K. Matz-Rensing, F. Schafer, T. Schneider, V. ter Meulen, and J. G. Muller. 2003. Impact of simian immunodeficiency virus (SIV) infection on lymphocyte numbers and T-cell turnover in different organs of rhesus monkeys. Blood 101:1213-1219. [DOI] [PubMed] [Google Scholar]
- 47.Staprans, S. I., P. J. Dailey, A. Rosenthal, C. Horton, R. M. Grant, N. Lerche, and M. B. Feinberg. 1999. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J. Virol. 73:4829-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veazey, R., B. Ling, I. Pandrea, H. McClure, A. Lackner, and P. Marx. 2003. Decreased CCR5 expression on CD4+ T cells of SIV-infected sooty mangabeys. AIDS Res. Hum. Retrovir. 19:227-233. [DOI] [PubMed] [Google Scholar]
- 49.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 50.Veazey, R. S., P. J. Klasse, T. J. Ketas, J. D. Reeves, M. Piatak, Jr., K. Kunstman, S. E. Kuhmann, P. A. Marx, J. D. Lifson, J. Dufour, M. Mefford, I. Pandrea, S. M. Wolinsky, R. W. Doms, J. A. DeMartino, S. J. Siciliano, K. Lyons, M. S. Springer, and J. P. Moore. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J. Exp. Med. 198:1551-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2003. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J. Infect. Dis. 187:769-776. [DOI] [PubMed] [Google Scholar]
- 53.Viollet, L., V. Monceaux, F. Petit, R. H. Fang, M. C. Cumont, B. Hurtrel, and J. Estaquier. 2006. Death of CD4+ T cells from lymph nodes during primary SIVmac251 infection predicts the rate of AIDS progression. J. Immunol. 177:6685-6694. [DOI] [PubMed] [Google Scholar]
- 54.Walker, B. D., and P. J. Goulder. 2000. AIDS. Escape from the immune system. Nature 407:313-314. [DOI] [PubMed] [Google Scholar]
- 55.Watson, A., J. Ranchalis, B. Travis, J. McClure, W. Sutton, P. R. Johnson, S. L. Hu, and N. L. Haigwood. 1997. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J. Virol. 71:284-290. [DOI] [PMC free article] [PubMed] [Google Scholar]