Abstract
The envelope glycoprotein of the Junín arenavirus (GP-C) mediates entry into target cells through a pH-dependent membrane fusion mechanism. Unlike other class I viral fusion proteins, the mature GP-C complex retains a cleaved, 58-amino-acid signal peptide (SSP) as an essential subunit, required both for trafficking of GP-C to the cell surface and for the activation of membrane fusion. SSP has been shown to associate noncovalently in GP-C via the cytoplasmic domain (CTD) of the transmembrane fusion subunit G2. In this report we investigate the molecular basis for this intersubunit interaction. We identify an invariant series of six cysteine and histidine residues in the CTD of G2 that is essential for incorporation of SSP in the GP-C complex. Moreover, we show that a CTD peptide fragment containing His-447, His-449, and Cys-455 specifically binds Zn2+ at subnanomolar concentrations. Together, these results suggest a zinc finger-like domain structure in the CTD of G2. We propose that the remaining residues in the series (His-459, Cys-467, and Cys-469) form an intersubunit zinc-binding center that incorporates Cys-57 of SSP. This unusual motif may act to retain SSP in the GP-C complex and position the ectodomain loop of SSP for its role in modulating membrane fusion activity. The unique tripartite organization of GP-C could provide novel molecular targets for therapeutic intervention in arenaviral disease.
Arenaviruses are endemic to rodent populations, and zoonotic infection of humans can result in severe acute hemorrhagic fevers (43, 49). Up to 300,000 infections by Lassa fever virus occur annually in Africa (44), and cases of Argentine, Bolivian, and Venezuelan hemorrhagic fevers are regularly reported in South America (49). Genetic diversification and worldwide dissemination of arenaviruses with their rodent hosts (51) provide opportunities for the continued emergence of new pathogenic strains (10). Vaccines to prevent arenavirus infection are not available, and treatment options for arenaviral hemorrhagic fevers are extremely limited. Ribavirin, a nonspecific antiviral agent with an incompletely understood mechanism of action, is currently used in severe and life-threatening cases with mixed results (60). In the case of Argentine hemorrhagic fever caused by infection with Junín virus (JUNV), early administration of human convalescent antiserum is the treatment of choice (17). There is an urgent need for effective vaccines and therapeutic agents to combat endemic arenavirus infections and to address biodefense concerns.
The arenaviruses are enveloped RNA viruses that encode ambisense expression of four viral proteins (8). The nucleoprotein (N) and RNA-dependent RNA polymerase (L) associate with the two single-strand genomic RNA segments to form the ribonucleoprotein core. Virion assembly occurs at the plasma membrane (45) and is promoted by the matrix protein (Z) (48, 59). Virus entry into the target cell is initiated by binding of the envelope glycoprotein (GP-C) to a cell surface receptor: α-dystroglycan in the Old World viruses (9, 34, 58) and transferrin receptor 1 in the New World hemorrhagic fever viruses (50). Upon binding, the virion particle is endocytosed (7), and fusion with cellular membrane is subsequently activated by acidic pH in the maturing endosome (11). As with other class I viral fusion proteins (12, 13, 28, 29, 56), this membrane fusion reaction is mediated by a series of conformational changes in GP-C that ultimately lead to formation of a stable six-helix bundle structure (18, 21, 63). Intervention strategies that target the steps in virus entry offer promise for effective antiviral therapeutics. Indeed, small-molecule arenavirus-specific entry inhibitors have recently been discovered (5).
GP-C is unique among class I envelope glycoproteins in that the mature complex retains its cleaved signal peptide as an essential element, together with the receptor-binding (G1) and transmembrane fusion (G2) subunits. The stable, 58-residue signal peptide (SSP) was initially identified as a component of the Old World arenavirus GP-C complex (16, 19, 35) and found to be essential for proteolytic maturation of the glycoprotein precursor in the Golgi body (1, 15, 35). Without SSP, the G1-G2 precursor is specifically retained in the endoplasmic reticulum (ER), in part through dibasic ER retrieval signals in the cytoplasmic domain (CTD) of G2 (1). This acts as a quality control mechanism to ensure that only the mature tripartite GP-C complex is transported to the cell surface for virion assembly. Unexpectedly, SSP incorporation in the GP-C complex also plays a crucial role in the activation of pH-dependent membrane fusion activity (65). Furthermore, the unusual interplay between the ectodomains of SSP and G2 in membrane fusion appears vulnerable to interference by the newly discovered small-molecule entry inhibitors (reference 5 and unpublished results). In this report, we provide biochemical and genetic evidence for a high-affinity zinc-binding domain (ZBD) in the CTD of G2 and discuss the implications of this novel structure for SSP association in GP-C and its membrane fusion activity.
MATERIALS AND METHODS
GP-C expression and MAbs.
GP-C from the pathogenic JUNV strain MC2 (24) was expressed in Vero cells by cotransfection of pcDNA3.1 (Invitrogen) plasmids encoding CD4sp-GPC (in which SSP is replaced by the conventional signal peptide of CD4) and SSP-term (in which a stop codon is introduced following the C-terminal SSP amino acid T58) (65). These components associate in trans and reconstitute the native GP-C complex (15). Transient expression utilized a recombinant vaccinia virus expressing T7 RNA polymerase (vTF7-3 [20]) and the T7 promoter of pcDNA3.1. The vaccinia virus-based β-galactosidase fusion reporter assay (46) was used to characterize the ability of GP-C to mediate pH-dependent cell-cell fusion (65, 66). Mutations in GP-C were introduced by QuikChange mutagenesis (Stratagene), and all constructs were verified by DNA sequencing. The murine monoclonal antibody (MAb) BF11 (53), directed to the G1 subunit of GP-C, was contributed by Tom Ksiasek and Tony Sanchez (Special Pathogens Branch, CDC, Atlanta, GA) and obtained through the NIH Biodefense and Emerging Infections Research Resources Repository.
GP-C expression was detected by immunoprecipitation of metabolically labeled complexes (65, 66). Briefly, cells were metabolically labeled using 50 μCi/ml of 35S-ProMix (Amersham Pharmacia Biotech) for 12 to 16 h. Cultures were washed in physiological buffered saline and lysed using cold Tris-saline buffer (50 mM Tris-HCl and 150 mM NaCl at pH 7.5) containing 1% Triton X-100 nonionic detergent and protease inhibitors (1 μg/ml each of aprotinin, leupeptin, and pepstatin). The expressed glycoproteins were immunoprecipitated from cleared lysates using MAb BF11 and protein A-Sepharose (Sigma). Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using NuPAGE 4 to 12% Bis-Tris gels (Invitrogen) and the recommended sample buffer containing reducing agent. In some cases, the glycoproteins are deglycosylated by treatment with peptide-N-glycosidase F (New England Biolabs) to facilitate resolution of the G1 and G2 subunits. Molecular size markers included 14C-methylated Rainbow proteins (Amersham Pharmacia Biotech). Radiolabeled proteins were visualized by phosphorimaging (Fuji FLA-3000G) and analyzed using ImageGauge software (Fuji).
Purification of chimeric MBP.
The sequence corresponding to the entire CTD of G2 (encoding P445 to H485) was adapted by PCR and appended in frame at the C terminus of maltose binding protein (MBP) using the KpnI polylinker site in the plasmid pMAL-c2E (New England Biolabs), to generate pMBP-ZBD. Expression of the chimeric MBP-ZBD protein in Escherichia coli K-12 strain TB1 (New England Biolabs) was induced by the addition of isopropyl-β-thiogalactopyranoside (IPTG) as recommended by New England Biolabs. Cell pellets were frozen overnight and subsequently lysed using Bugbuster protein extraction reagent (primary amine free; Novagen) in the presence of protease inhibitors (1 μg/ml each of aprotinin, leupeptin, and pepstatin). Cleared extracts were incubated with amylose resin (New England Biolabs) in buffer containing 20 mM HEPES (pH 6.8) and 200 mM NaCl for 2 h at 4°C with rocking. The resin was then packed into a column and washed extensively with this buffer. Bound MBP chimera was eluted by the addition of 10 mM maltose. The yield of purified protein was 2 to 3 mg per g of wet cells.
Protein characterization.
Proteins were analyzed by SDS-PAGE and visualized using SYPRO Red protein stain (Molecular Probes). Molecular size markers included Mark12 standards (Invitrogen). Protein concentrations were determined by the Coomassie Plus assay (Pierce) and corresponded to those measured by optical density at 280 nm in 6 M GuHCl (14). Zinc content was determined using the 4-(2-pyridylaxo)resorcinol (Aldrich) reagent in 4 M GuHCl (30, 42).
Equilibrium binding studies.
Prior to use for zinc binding, the purified proteins were dialyzed into buffer containing 50 mM HEPES (pH 6.8) and 20 mM NaCl (HEPES-NaCl buffer) and incubated with an excess of tris(2-carboxyethyl)phosphine (TCEP; Pierce) for 20 min at room temperature in order to reduce any disulfide bonds that form on exposure to air and in the absence of zinc. TCEP does not bind metal ions and is thus uniquely suited for these studies (23, 32). We used 1 mM TCEP for 10 μM protein and 0.1 mM for 200 nM protein. These concentrations of TCEP were maintained throughout the subsequent binding experiment. Radioactive zinc (65Zn) was obtained from Brookhaven National Laboratory (U.S. Department of Energy) as a 2 mM solution of ZnCl2 in 0.1 N HCl. The specific activity is 2 Ci/mmol (batch Zn65-012506). Equilibrium dialysis studies were performed using a modified method in which unbound ligand is determined by centrifugal ultrafiltration (26, 37). In a typical study, 200 nM of MBP-ZBD protein is incubated at room temperature with 10 nM of neat 65Zn2+ in 5.0 ml of HEPES-NaCl buffer containing 0.1 mM TCEP. Equilibrium binding is attained within 60 min (not shown). At this time, the reaction mixture is sampled to determine the total amount of 65Zn2+, and the free 65Zn2+ is determined by ultrafiltration (Amicon Ultra-4; 10,000-molecular-weight cutoff). Based on the specific radioactivity of the reaction mixture (cpm/ml for the known total concentration of Zn2+), the concentration of free and bound zinc is determined. Samples were counted using a Captus multichannel analyzer (Capintec) and a single-well detector (2-ml capacity). The high-energy gamma emission of 65Zn (950 to 1,200 keV) was detected at ∼2.5% efficiency. Using this methodology, we were effectively limited to detecting zinc binding at concentrations of ≥10 nM Zn2+ (i.e., ≥2,000 cpm per 2-ml sample).
Competitive metal ion binding studies.
The specificity and selectivity of metal ion binding were determined in a 5-ml equilibrium binding reaction mixture containing 200 nM MBP-ZBD, 10 nM 65Zn2+, and a 10- to 10,000-fold excess of CdCl2, CoCl2, CuCl2, MgCl2, or cold ZnCl2. A 50% inhibitory concentration (IC50) for each metal ion was defined as that which competes for 50% of 65Zn2+ binding. Because of the excess of MBP-ZBD over 65Zn2+ in the reaction, the relative IC50 is reported as a ratio of the competitor IC50 compared to that of cold Zn2+.
RESULTS
Conserved cysteine and histidine residues in the CTD of G2.
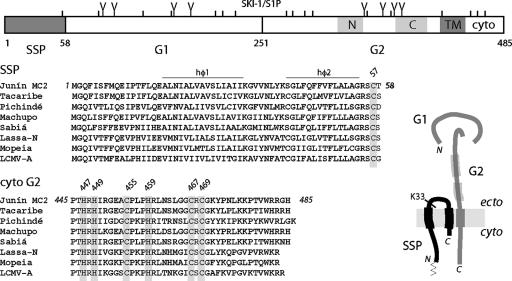
Previous studies have demonstrated that the CTD of G2 is essential for SSP incorporation in the mature GP-C complex (1). GP-C mutants bearing deletions in this region are unable to bind SSP, while chimeric proteins containing only the transmembrane domain and CTD of G2 associate with and require SSP for transit through the Golgi body (1). To examine the role of the G2 CTD in the assembly of the tripartite GP-C complex, we compared amino acid sequences in the New World and Old World arenaviruses (Fig. 1). Inspection of the CTD revealed a well-conserved N-terminal region (P445 through G470) and a variable C-terminal region (K471 through H485) that includes dibasic ER retrieval motifs (1). Strikingly, the N-terminal region contains a series of six invariantly conserved cysteine and histidine residues (Fig. 1). This array (H447, H449, C455, H459, C467, and C469) was suggestive of the clustered cysteine and histidine sequences found in many zinc finger proteins (27, 31, 33, 38).
FIG. 1.
The tripartite GP-C complex. A representation of the JUNV GP-C open reading frame is shown on top. Amino acids are numbered from the initiating methionine, and cysteine residues ( ) and potential glycosylation sites (
) and potential glycosylation sites ( ) are marked. The signal peptidase and SKI-1/S1P (4, 35, 39) cleavage sites, and the resulting SSP, G1, and G2 subunits, are indicated. Within G2, the C-terminal transmembrane domain (TM) and CTD (cyto) are marked, as are the N- and C-terminal heptad-repeat regions in the ectodomain (light gray shading). Below, we show a comparison of arenavirus amino acid sequences in SSP and the CTD of G2. Sequences are from New World isolates Junín (D10072), Tacaribe (M20304), Pichindé (U77601), Machupo (AY129248), and Sabiá (YP_089665) and Old World isolates Lassa-Nigeria (X52400), Mopeia (M33879), and LCMV-Armstrong (M20869). The array of cysteine and histidine residues is highlighted in gray and numbered, and the two hydrophobic, membrane-spanning regions in SSP (hφ1 and hφ2) are marked. A schematic drawing of the tripartite GP-C complex is shown at the lower right. The model depicts the bitopic membrane topology of SSP in relation to the CTD of G2 (2). The myristoylation site (66) and fusion-critical Lys-33 residue (65) in SSP are indicated, as are the heptad-repeat regions in the G2 ectodomain (thicker lines). The N or C termini of the subunits are labeled. The drawing is not to scale.
) are marked. The signal peptidase and SKI-1/S1P (4, 35, 39) cleavage sites, and the resulting SSP, G1, and G2 subunits, are indicated. Within G2, the C-terminal transmembrane domain (TM) and CTD (cyto) are marked, as are the N- and C-terminal heptad-repeat regions in the ectodomain (light gray shading). Below, we show a comparison of arenavirus amino acid sequences in SSP and the CTD of G2. Sequences are from New World isolates Junín (D10072), Tacaribe (M20304), Pichindé (U77601), Machupo (AY129248), and Sabiá (YP_089665) and Old World isolates Lassa-Nigeria (X52400), Mopeia (M33879), and LCMV-Armstrong (M20869). The array of cysteine and histidine residues is highlighted in gray and numbered, and the two hydrophobic, membrane-spanning regions in SSP (hφ1 and hφ2) are marked. A schematic drawing of the tripartite GP-C complex is shown at the lower right. The model depicts the bitopic membrane topology of SSP in relation to the CTD of G2 (2). The myristoylation site (66) and fusion-critical Lys-33 residue (65) in SSP are indicated, as are the heptad-repeat regions in the G2 ectodomain (thicker lines). The N or C termini of the subunits are labeled. The drawing is not to scale.
Zinc fingers are compact protein domains that coordinate one or more zinc ions (31). Tetrahedral ligation of Zn2+ serves to stabilize protein folds and, in some cases, interprotein interactions (22, 27, 40). Structurally diverse, zinc fingers are found in cellular proteins that function in DNA replication and repair, transcriptional regulation, protein modification and degradation, metabolism and signaling, vesicular trafficking, and apoptosis (22, 31, 33, 38). It has been estimated that zinc finger proteins represent 2% of the human genome coding capacity (41). Zinc fingers are classified according to the amino acids involved in zinc coordination (predominantly cysteines and histidines) and by the size, topology, and folding of the intervening loops (27, 33). The array of cysteine and histidine residues in G2 generally conforms to the sequence motifs in known zinc fingers, although the identical pattern is not represented in existing databases (InterPro database, European Bioinformatics Institute). These considerations led us to investigate the zinc-binding properties of the CTD.
Zinc-binding activity of the G2 CTD.
To characterize zinc binding, we appended the entire CTD sequence (P445 to H485) (Fig. 1) to the C terminus of MBP (New England Biolabs). The chimeric MBP-ZBD protein was expressed in E. coli and purified using amylose resin affinity chromatography (Fig. 2). No Zn2+ was added during the purification procedure, and the isolated protein was devoid of zinc (<0.03 mol per mol of protein), as determined by reaction with 4-(2-pyridylazo)resorcinol (42). We also generated a mutant chimera (MBP-noZBD) in which the conserved CTD cysteine and histidine residues were all replaced by amino acids that do not coordinate zinc ion (27). The three cysteines were changed to serine, a similarly sized side chain that differs only at the hydroxyl oxygen, whereas the three histidines were replaced by asparagine, another nitrogen-containing side chain. The MBP-noZBD protein was used to assess nonspecific zinc binding and the potential role of the cysteine-histidine sequence motif.
FIG. 2.
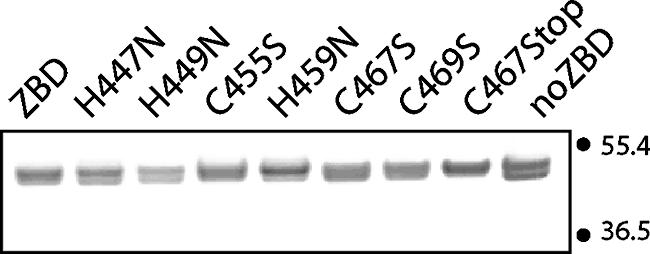
Purified MBP chimeras containing the CTD of G2. The purity of the chimeric proteins (500 ng each) was assessed using SDS-PAGE. Wild-type MBP-ZBD and the individual mutants are indicated. C467Stop contains a stop codon at position 467, and MBP-noZBD includes the three cysteine and three histidine mutations. The isoforms seen in the samples vary between analyses and likely reflect different conformational states of the protein. Numbers at right are molecular masses in kilodaltons.
Binding of radioactive 65Zn2+ was measured by a modified equilibrium dialysis method in which centrifugal ultrafiltration is used to determine the concentration of free ligand (26, 37). In initial experiments, we incubated 10 μM of MBP-ZBD with 100 nM 65ZnCl2 (Brookhaven National Laboratory; 2 Ci/mmol) in 5.0 ml of HEPES-NaCl buffer containing 1 mM TCEP, a nonthiol reducing agent that does not chelate metal ions (23, 32). After 60 min of incubation with MBP-ZBD, less than 1% of the input zinc radioactivity was found as free Zn2+ in the ultrafiltrate (Fig. 3, left), indicating complete binding. In contrast, greater than 90% of the input Zn2+ from the MBP-noZBD binding reaction was free. Our results indicate that the CTD of G2 can bind Zn2+ and that binding is dependent on the intact array of cysteine and histidine residues. Furthermore, all the available Zn2+ was bound at a zinc concentration of 100 nM. In other experiments using higher concentrations of Zn2+, binding reached a plateau of ∼0.3 mol zinc per mol of protein (not shown). The substoichiometric amount of zinc binding may reflect oxidative damage to the cysteines.
FIG. 3.
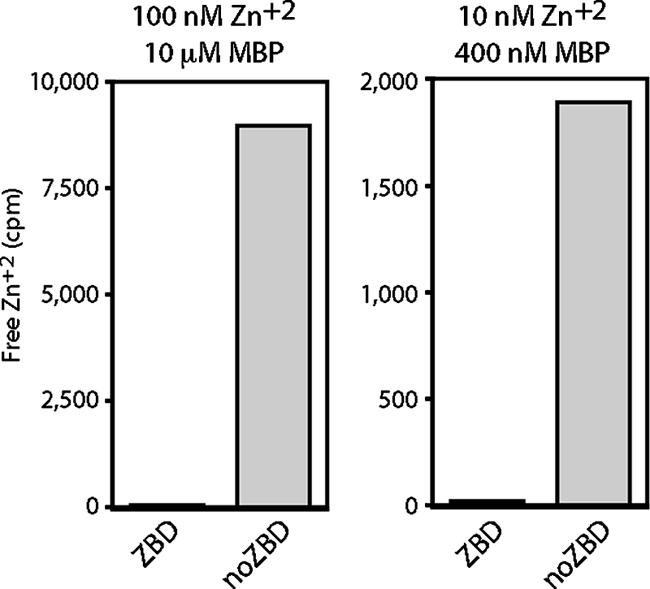
Specific binding of zinc ion to MBP-ZBD. MBP-ZBD and MBP-noZBD (10 μM, left, and 400 nM, right) were incubated with 100 μΜ and 10 nM 65Zn2+, respectively, as described in the text. Free 65Zn2+ was determined by ultrafiltration. The counts per minute (cpm) of free 65Zn2+ in the ultrafiltrate (1-ml or 2-ml sample, respectively) are plotted. The total radioactivity in the binding reaction is used as the full-scale value in each graph (10,000 and 2,000 cpm, respectively) to demonstrate the relative proportion of unbound zinc.
Efforts to attain subsaturating concentrations of Zn2+ and determine an apparent dissociation constant (Kd) were ultimately limited by the relatively low specific activity of the zinc isotope and the high affinity of binding (see Materials and Methods). At the lowest practical zinc concentration (10 nM 65Zn2+), binding by the MBP-ZBD protein remained quantitative even at low (200 to 400 nM) protein concentrations (Fig. 3, right). Based on the limits of the assay, we can conclude that the Kd for Zn2+ binding to MBP-ZBD is likely to be significantly less than 1 nM (see below). The high-affinity interaction of MBP-ZBD with Zn2+ is consistent with zinc finger binding (3).
The ability of MBP-ZBD to bind other divalent transition-metal ions was determined by equilibrium dialysis using 10 nM 65Zn2+ and an excess of Cd2+, Co2+, or Cu2+. The concentration of competitor ion required to inhibit Zn2+ binding by 50% (IC50) was used to assess the relative binding affinity. The selectivity of binding to MBP-ZBD was determined as: Zn2+ > Cu2+ > Cd2+ ≫ Co2+. This order of preference conforms to that of well-characterized zinc finger proteins (3, 36). The respective IC50s for Cd2+, Co2+, and Cu2+ were 10-, 450-, and 5-fold that of Zn2+. Mg2+ did not compete for binding at concentrations up to 100 μM, demonstrating the requirement for a transition-metal ion and the specificity of zinc binding.
Amino acid requirements for zinc-binding activity.
To determine the role of specific cysteine and histidine side chains in zinc binding, we introduced the respective serine and asparagine mutations individually into MBP-ZBD. The mutant proteins were affinity purified (Fig. 2), and 400 nM of each was used to determine binding with 10 nM 65Zn2+ (Fig. 4, top). To enhance the distinctions among the mutants, the assay was also performed using 200 nM protein (Fig. 4, bottom). Under these conditions, zinc binding was reduced markedly by the C455S mutation and to a lesser extent by H447N and H449N (Fig. 4). These results extrapolate to dissociation constants of 12 nM for C455S and 1 to 2 nM for H447N and H449. We infer that these three membrane-proximal residues (H447, H449, and C455) are required for high-affinity binding of Zn2+ by MBP-ZBD. Each is capable of coordinating zinc ion or may indirectly contribute to formation of the properly folded zinc-binding center. Interestingly, mutations in the three distal residues in the array (H459N, C467S, and C469S) had little or no detectable effect on zinc binding in this assay.
FIG. 4.
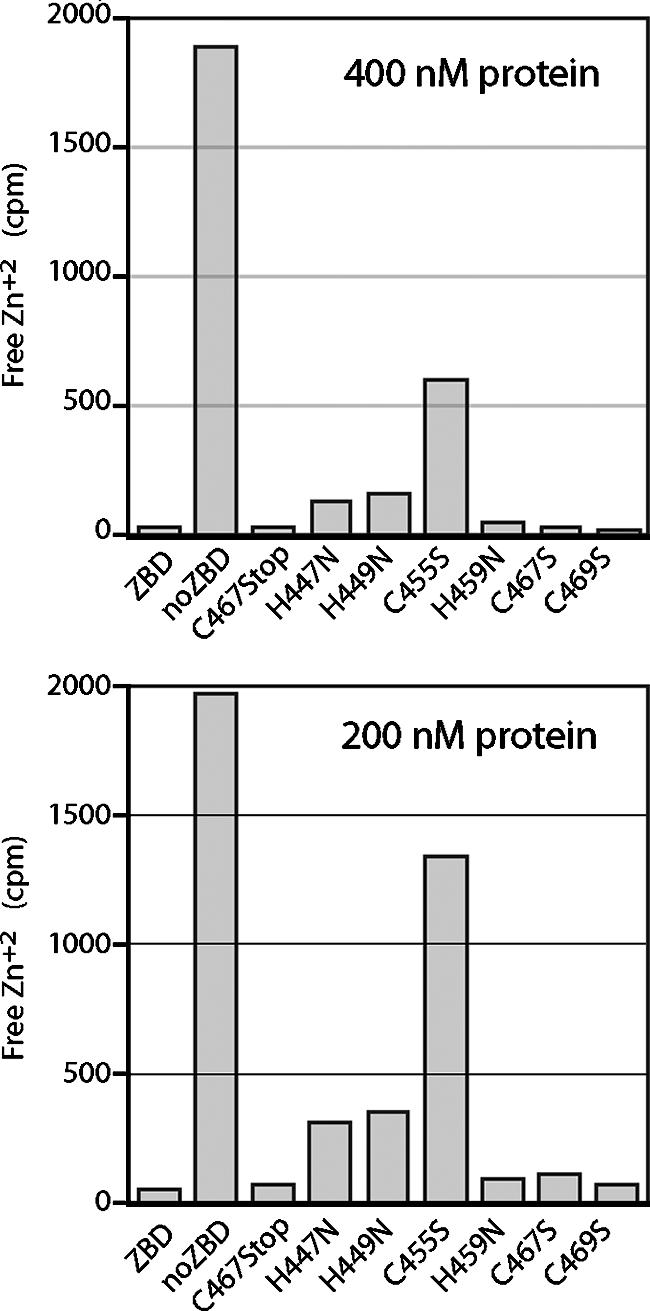
Zinc binding by MBP-ZBD mutants. Wild-type and mutant MBP-ZBD proteins (400 nM in top panel and 200 nM in bottom panel) were incubated with 10 nM 65Zn2+ as described in the text. The cpm of free Zn2+ are plotted, and the full scale (2,000 cpm) represents 10 nM Zn2+. The results shown here are representative of six independent studies.
To further dissect the sequence requirements for zinc binding, we truncated MBP-ZBD at position 467 to delete the two C-terminal cysteines. On equilibrium dialysis, this C467Stop mutant was indistinguishable from the wild-type protein in its zinc binding (Fig. 4). Taken together with the wild-type binding by the H459N mutant (above), we conclude that the membrane-proximal region of the array including H447, H449, and C455 can fold autonomously to bind zinc ion with high affinity.
Conserved cysteines and histidines are required for SSP incorporation in GP-C.
The functional significance of zinc binding and the conserved residues in GP-C was determined by individually replacing each cysteine and histidine residue in the CTD with serine or asparagine, respectively. These mutations were introduced into a GP-C construct (CD4sp-GPC) that utilizes the conventional signal peptide of human CD4 for expression of the G1-G2 precursor protein (1). Coexpression of wild-type CD4sp-GPC in trans with an SSP polypeptide has been shown to reconstitute the native GP-C complex (1, 15, 64). This trans-complementation method was chosen to obviate experimental concerns regarding possible effects of the mutations on signal peptidase cleavage of full-length GP-C. The GP-C complexes were metabolically labeled with 35S-ProMix (Amersham Pharmacia Biotech) and immunoprecipitated using G1-specific MAb BF11 (53). SDS-PAGE analysis of the wild-type GP-C complex reveals the association with SSP (Fig. 5, top) and the proteolytic maturation of the G1-G2 precursor (Fig. 5, bottom). In contrast to the wild-type CD4sp-GPC, none of the single cysteine and histidine mutants was able to associate with SSP in trans (Fig. 5, top). Proteolytic maturation of the G1-G2 precursor was also absent in the mutants (Fig. 5, bottom), presumably reflecting the inability of the precursor to transit through the Golgi body in the absence of SSP (1). As anticipated, the mutants were unable to support membrane fusion (Fig. 6). Thus, CTD residues that are dispensable for high-affinity zinc binding (H459, C467, and C469) as well as those that are required (H447, H449, and C455) are each essential for SSP incorporation in the GP-C complex.
FIG. 5.
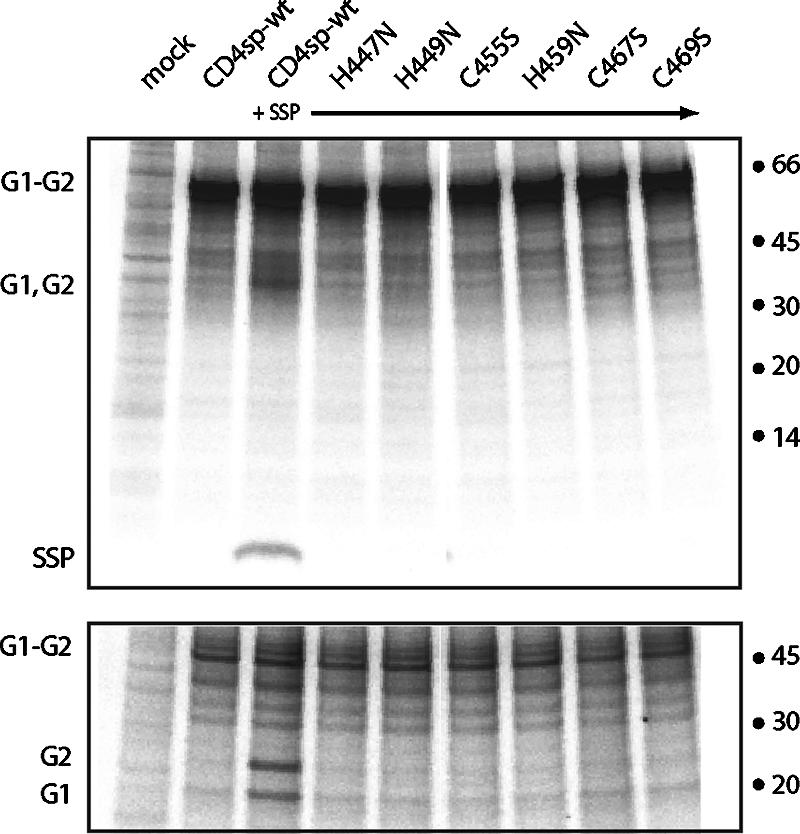
SSP association in GP-C complexes bearing mutations in the G2 cysteine-histidine array. Vero cells were transfected to express wild-type and mutant CD4sp-GPC in trans with wild-type SSP (+SSP) (65). Metabolically labeled complexes were immunoprecipitated using G1-specific MAb BF11 (53) and separated on NuPAGE (Invitrogen) 4 to 12% Bis-Tris gels under denaturing and reducing conditions (top panel). The image has been darkened in order to visualize SSP. The mature G1 and G2 subunits are not as well resolved as the glycoproteins and are rendered distinct on deglycosylation by peptide-N-glycosidase F (lower panel). The G1-G2 precursor and GP-C subunits are labeled, as well as the 14C-labeled protein markers (in kilodaltons) (Amersham Biosciences).
FIG. 6.
Membrane fusion activity of mutant GP-C complexes. pH-dependent cell-cell fusion by the trans-complemented GP-C complex was initiated by a pulse of medium adjusted to pH 5.0 and detected using the recombinant vaccinia virus-based β-galactosidase reporter assay (46) as previously described (65, 66). β-Galactosidase expression was quantitated using the chemiluminescent substrate GalactoLite Plus (Tropix), and the percentage of fusion relative to the wild-type CD4sp-GPC complex (CD4sp + SSP) is indicated. Error bars represent ±1 standard deviation.
Taken together, our studies provide strong biochemical evidence for a cysteine- and histidine-containing zinc-finger-like motif in the CTD of G2 and for its essential role in SSP incorporation in the tripartite GP-C complex. Although many viruses have adopted zinc fingers in their structural, regulatory, and catalytic proteins (25, 47, 57, 62, 67), the motif has not been described in a virus envelope glycoprotein. In addition to its involvement in SSP association, the unusual ZBD in G2 protein may participate in functions typically served by the CTD of viral envelope glycoproteins, including roles in the intracellular trafficking and membrane fusion activity of GP-C and in virion assembly and budding.
DISCUSSION
We have identified a novel zinc-binding activity in the CTD of the arenavirus G2 fusion protein. An array of six cysteine and histidine residues in the CTD is uniformly conserved in New World and Old World arenaviruses, and peptide fragments containing the three membrane-proximal residues (H447, H449, and C455) specifically bind Zn2+ at subnanomolar concentrations. Our results indicate that this region of the cysteine-histidine array in the CTD of G2 can fold to form a zinc-finger-like structural domain.
The three distal residues in the array (H459, C467, and C469) are not required for high-affinity zinc binding. Nonetheless, all six residues in the array are essential for SSP incorporation in the mature GP-C complex; mutations at any of these positions prevent association and render the envelope glycoprotein defective. This shared phenotype may of course arise through a variety of mutational effects. Interestingly, however, SSP association in GP-C is also abolished by mutation at an absolutely conserved cysteine residue (C57) in the C-terminal CTD of SSP (54, 64). Based on the bitopic membrane topology of SSP in which both N and C termini reside in the cytosol (2) (Fig. 1), this C-terminal region must be translocated from the ER lumen following signal peptidase cleavage. With the exception of the inviolable C57, other residues in this short region (R55SCT58 in JUNV) are not conserved among the arenaviruses, and each can be mutated without effect on SSP association (64). In light of the shared phenotype in mutants at C57 in SSP and within the cysteine-histidine array of G2, we speculate that H459, C467, and C469 may participate together with C57 in forming an intersubunit zinc-binding center, possibly in conjunction with the membrane-proximal zinc center. Although translocation of C57 into the cytosol is independent of G2 (2), formation of a zinc finger motif bridging SSP and G2 may be needed to stabilize SSP incorporation in the GP-C complex.
We have shown that peptide fragments including the membrane-proximal H447, H449, and C455 residues can bind Zn2+ with extremely high affinity and that single-amino-acid substitutions at these positions substantially reduce affinity to a range that is consistent with the effects of single coordination-site mutations in known zinc fingers (6, 55). The residual nanomolar affinity observed in these triply ligated mutants, however, might suggest that zinc binding by the three distal cysteine and histidine residues would also be detectable. The lack of binding in our studies may simply reflect the inability of these residues to coordinate zinc or the oxidative lability of the cysteines in the recombinant peptide model in MBP-ZBD. Alternatively, SSP may be important for zinc binding and formation of the proposed intersubunit zinc center. Because the C-terminal region of SSP is short and otherwise permissive to amino acid changes (64), it appears that proper placement of the coordinating C57 side chain is promoted by additional sequence elements that lie in the N-terminal region of SSP (54) or the membrane-spanning domains of SSP and G2. Indeed, mutations in the second transmembrane domain of SSP that prevent SSP association (2) might do so by perturbing the positioning of C57 and thereby preventing formation of the intersubunit zinc center. Structural studies are needed in order to further assess the potential for zinc binding by H459, C467, and C469 and the proposed participation of C57 in SSP.
We should emphasize that specific interactions between SSP and G2 are required for the membrane fusion activity of GP-C. Recent studies have shown that mutations that diminish positive charge at K33 in the ectodomain loop of SSP increase the H+ concentration needed for the activation of membrane fusion (65). These results suggest that that K33 interacts with titratable residues in the ectodomain of G2 to modulate fusion activation at acidic pH (65). Formation of a zinc-finger-like structure on the cytosolic face of GP-C may be important in positioning K33 and the ectodomain loop of SSP for this role. In this regard, the localized interaction between SSP and the G2 ectodomain in membrane fusion may provide a tractable model for studying pH-induced activation of viral fusion and the structural determinants on either side of the membrane that control this fundamental function of virus envelope glycoproteins.
In proposing an intersubunit ZBD, we suggest a molecular basis for the retention and positioning of SSP in the GP-C complex for its role in pH-dependent membrane fusion. With this report, the three structural proteins of the arenaviruses are now known to contain a ZBD (52, 61). Biophysical and structural studies of the unusual ZBD in GP-C are needed to define the zinc centers and characterize the requirements for folding. A detailed understanding of the structure and function of the unique SSP subunit in GP-C may suggest novel opportunities for therapeutic intervention in arenavirus infection and hemorrhagic fevers.
Acknowledgments
We are grateful to Michele McGuirl and Michael Machczynski (The University of Montana) for their guidance in bioinorganic protein chemistry and to Min Lu (Weill Medical College of Cornell University) and Meg Trahey (The University of Montana) for critical review of the manuscript. We thank James R. Thomas and the St. Patrick Hospital and Health Sciences Center (Missoula, MT) for generous access to a multichannel gamma counter and the NIH Biodefense and Emerging Infections Research Resources Repository for providing MAbs.
This work was supported by NIH research grant AI059355 and a subaward from the Rocky Mountain Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (NIH grant U54 AI065357).
Footnotes
Published ahead of print on 10 October 2007.
REFERENCES
- 1.Agnihothram, S. S., J. York, and J. H. Nunberg. 2006. Role of the stable signal peptide and cytoplasmic domain of G2 in regulating intracellular transport of the Junin virus envelope glycoprotein complex. J. Virol. 80:5189-5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agnihothram, S. S., J. York, M. Trahey, and J. H. Nunberg. 2007. Bitopic membrane topology of the stable signal peptide in the tripartite Junín virus GP-C envelope glycoprotein complex. J. Virol. 81:4331-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg, J. M., and D. L. Merkle. 1989. On the metal specificity of “zinc finger” proteins. J. Am. Chem. Soc. 111:3759-3761. [Google Scholar]
- 4.Beyer, W. R., D. Popplau, W. Garten, D. von Laer, and O. Lenz. 2003. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 77:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolken, T. C., S. Laquerre, Y. Zhang, T. R. Bailey, D. C. Pevear, S. S. Kickner, L. E. Sperzel, K. F. Jones, T. K. Warren, S. A. Lund, D. L. Kirkwood-Watts, D. S. King, A. C. Shurtleff, M. C. Guttieri, Y. Deng, M. Bleam, and D. E. Hruby. 2006. Identification and characterization of potent small molecule inhibitor of hemorrhagic fever New World arenaviruses. Antivir. Res. 69:86-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bombarda, E., H. Cherradi, N. Morellet, B. P. Roques, and Y. Mely. 2002. Zn2+ binding properties of single-point mutants of the C-terminal zinc finger of the HIV-1 nucleocapsid protein: evidence of a critical role of cysteine 49 in Zn2+ dissociation. Biochemistry 41:4312-4320. [DOI] [PubMed] [Google Scholar]
- 7.Borrow, P., and M. B. A. Oldstone. 1994. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology 198:1-9. [DOI] [PubMed] [Google Scholar]
- 8.Buchmeier, M. J., M. D. Bowen, and C. J. Peters. 2001. Arenaviruses and their replication, p. 1635-1668. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 9.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. A. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2000. Fatal illnesses associated with a new world arenavirus—California, 1999-2000. Morb. Mortal. Wkly. Rep. 49:709-711. [PubMed] [Google Scholar]
- 11.Di Simone, C., M. A. Zandonatti, and M. J. Buchmeier. 1994. Acidic pH triggers LCMV membrane fusion activity and conformational change in the glycoprotein spike. Virology 198:455-465. [DOI] [PubMed] [Google Scholar]
- 12.Earp, L. J., S. E. Delos, H. E. Park, and J. M. White. 2005. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 285:25-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 14.Edelhoch, H. 1967. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6:1948-1954. [DOI] [PubMed] [Google Scholar]
- 15.Eichler, R., O. Lenz, T. Strecker, M. Eickmann, H. D. Klenk, and W. Garten. 2003. Identification of Lassa virus glycoprotein signal peptide as a trans-acting maturation factor. EMBO Rep. 4:1084-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichler, R., O. Lenz, T. Strecker, and W. Garten. 2003. Signal peptide of Lassa virus glycoprotein GP-C exhibits an unusual length. FEBS Lett. 538:203-206. [DOI] [PubMed] [Google Scholar]
- 17.Enria, D. A., A. M. Briggiler, N. J. Fernandez, S. C. Levis, and J. I. Maiztegui. 1984. Importance of dose of neutralising antibodies in treatment of Argentine haemorrhagic fever with immune plasma. Lancet ii:255-256. [DOI] [PubMed] [Google Scholar]
- 18.Eschli, B., K. Quirin, A. Wepf, J. Weber, R. Zinkernagel, and H. Hengartner. 2006. Identification of an N-terminal trimeric coiled-coil core within arenavirus glycoprotein 2 permits assignment to class I viral fusion proteins. J. Virol. 80:5897-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froeschke, M., M. Basler, M. Groettrup, and B. Dobberstein. 2003. Long-lived signal peptide of lymphocytic choriomeningitis virus glycoprotein pGP-C. J. Biol. Chem. 278:41914-41920. [DOI] [PubMed] [Google Scholar]
- 20.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallaher, W. R., C. DiSimone, and M. J. Buchmeier. 2001. The viral transmembrane superfamily: possible divergence of Arenavirus and Filovirus glycoproteins from a common RNA virus ancestor. BMC Microbiol. 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamsjaeger, R., C. K. Liew, F. E. Loughlin, M. Crossley, and J. P. Mackay. 2007. Sticky fingers: zinc-fingers as protein-recognition motifs. Trends Biochem. Sci. 32:63-70. [DOI] [PubMed] [Google Scholar]
- 23.Getz, E. B., M. Xiao, T. Chakrabarty, R. Cooke, and P. R. Selvin. 1999. A comparison between the sulfhydryl reductants tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry. Anal. Biochem. 273:73-80. [DOI] [PubMed] [Google Scholar]
- 24.Ghiringhelli, P. D., R. V. Rivera-Pomar, M. E. Lozano, O. Grau, and V. Romanowski. 1991. Molecular organization of Junin virus S RNA: complete nucleotide sequence, relationship with other members of the Arenaviridae and unusual secondary structures. J. Gen. Virol. 72:2129-2141. [DOI] [PubMed] [Google Scholar]
- 25.Green, L. M., and J. M. Berg. 1989. A retroviral Cys-Xaa2-Cys-Xaa4-His-Xaa4-Cys peptide binds metal ions: spectroscopic studies and a proposed three-dimensional structure. Proc. Natl. Acad. Sci. USA 86:4047-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha, N. C., B. C. Oh, S. Shin, H. J. Kim, T. K. Oh, Y. O. Kim, K. Y. Choi, and B. H. Oh. 2000. Crystal structures of a novel, thermostable phytase in partially and fully calcium-loaded states. Nat. Struct. Biol. 7:147-153. [DOI] [PubMed] [Google Scholar]
- 27.Harding, M. M. 2004. The architecture of metal coordination groups in proteins. Acta Crystallogr. D Biol. Crystallogr. 60:849-859. [DOI] [PubMed] [Google Scholar]
- 28.Harrison, S. C. 2005. Mechanism of membrane fusion by viral envelope proteins. Adv. Virus Res. 64:231-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughson, F. M. 1997. Enveloped viruses: a common mode of membrane fusion? Curr. Biol. 7:R565-R569. [DOI] [PubMed] [Google Scholar]
- 30.Hunt, J. B., S. H. Neece, and A. Ginsburg. 1985. The use of 4-(2-pyridylazo)resorcinol in studies of zinc release from Escherichia coli aspartate transcarbamoylase. Anal. Biochem. 146:150-157. [DOI] [PubMed] [Google Scholar]
- 31.Klug, A., and J. W. Schwabe. 1995. Protein motifs 5. Zinc fingers. FASEB J. 9:597-604. [PubMed] [Google Scholar]
- 32.Krezel, A., R. Latajka, G. D. Bujacz, and W. Bal. 2003. Coordination properties of tris(2-carboxyethyl)phosphine, a newly introduced thiol reductant, and its oxide. Inorg. Chem. 42:1994-2003. [DOI] [PubMed] [Google Scholar]
- 33.Krishna, S. S., I. Majumdar, and N. V. Grishin. 2003. Structural classification of zinc fingers: survey and summary. Nucleic Acids Res. 31:532-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunz, S., P. Borrow, and M. B. A. Oldstone. 2002. Receptor structure, binding, and cell entry of arenaviruses. Curr. Top. Microbiol. Immunol. 262:111-137. [DOI] [PubMed] [Google Scholar]
- 35.Kunz, S., K. H. Edelmann, J.-C. de la Torre, R. Gorney, and M. B. A. Oldstone. 2003. Mechanisms for lymphocytic choriomeningitis virus glycoprotein cleavage, transport, and incorporation into virions. Virology 314:168-178. [DOI] [PubMed] [Google Scholar]
- 36.Lachenmann, M. J., J. E. Ladbury, J. Dong, K. Huang, P. Carey, and M. A. Weiss. 2004. Why zinc fingers prefer zinc: ligand-field symmetry and the hidden thermodynamics of metal ion selectivity. Biochemistry 43:13910-13925. [DOI] [PubMed] [Google Scholar]
- 37.Ladant, D. 1995. Calcium and membrane binding properties of bovine neurocalcin delta expressed in Escherichia coli. J. Biol. Chem. 270:3179-3185. [PubMed] [Google Scholar]
- 38.Laity, J. H., B. M. Lee, and P. E. Wright. 2001. Zinc finger proteins: new insights into structural and functional diversity. Curr. Opin. Struct. Biol. 11:39-44. [DOI] [PubMed] [Google Scholar]
- 39.Lenz, O., J. ter Meulen, H.-D. Klenk, N. G. Seidah, and W. Garten. 2001. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl. Acad. Sci. USA 98:12701-12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackay, J. P., and M. Crossley. 1998. Zinc fingers are sticking together. Trends Biochem. Sci. 23:1-4. [DOI] [PubMed] [Google Scholar]
- 41.Matthews, J. M., and M. Sunde. 2002. Zinc fingers—folds for many occasions. IUBMB Life 54:351-355. [DOI] [PubMed] [Google Scholar]
- 42.McCall, K. A., and C. A. Fierke. 2000. Colorimetric and fluorimetric assays to quantitate micromolar concentrations of transition metals. Anal. Biochem. 284:307-315. [DOI] [PubMed] [Google Scholar]
- 43.McCormick, J. B., and S. P. Fisher-Hoch. 2002. Lassa fever. Curr. Top. Microbiol. Immunol. 262:75-109. [DOI] [PubMed] [Google Scholar]
- 44.McCormick, J. B., P. A. Webb, J. W. Krebs, K. M. Johnson, and E. S. Smith. 1987. A prospective study of the epidemiology and ecology of Lassa fever. J. Infect. Dis. 155:437-444. [DOI] [PubMed] [Google Scholar]
- 45.Murphy, F. A., P. A. Webb, K. M. Johnson, S. G. Whitfield, and W. A. Chappell. 1970. Arenoviruses in Vero cells: ultrastructural studies. J. Virol. 6:507-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nussbaum, O., C. C. Broder, and E. A. Berger. 1994. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J. Virol. 68:5411-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paterson, R. G., G. P. Leser, M. A. Shaughnessy, and R. A. Lamb. 1995. The paramyxovirus SV5 V protein binds two atoms of zinc and is a structural component of virions. Virology 208:121-131. [DOI] [PubMed] [Google Scholar]
- 48.Perez, M., R. C. Craven, and J. C. de la Torre. 2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc. Natl. Acad. Sci. USA 100:12978-12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters, C. J. 2002. Human infection with arenaviruses in the Americas. Curr. Top. Microbiol. Immunol. 262:65-74. [DOI] [PubMed] [Google Scholar]
- 50.Radoshitzky, S. R., J. Abraham, C. F. Spiropoulou, J. H. Kuhn, D. Nguyen, W. Li, J. Nagel, P. J. Schmidt, J. H. Nunberg, N. C. Andrews, M. Farzan, and H. Choe. 2007. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 446:92-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salazar-Bravo, J., L. A. Ruedas, and T. L. Yates. 2002. Mammalian reservoirs of arenaviruses. Curr. Top. Microbiol. Immunol. 262:25-63. [DOI] [PubMed] [Google Scholar]
- 52.Salvato, M. S., K. J. Schweighofer, J. Burns, and E. M. Shimomaye. 1992. Biochemical and immunological evidence that the 11 kDa zinc-binding protein of lymphocytic choriomeningitis virus is a structural component of the virus. Virus Res. 22:185-198. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez, A., D. Y. Pifat, R. H. Kenyon, C. J. Peters, J. B. McCormick, and M. P. Kiley. 1989. Junin virus monoclonal antibodies: characterization and cross-reactivity with other arenaviruses. J. Gen. Virol. 70:1125-1132. [DOI] [PubMed] [Google Scholar]
- 54.Saunders, A. A., J. P. Ting, J. Meisner, B. W. Neuman, M. Perez, J. C. de la Torre, and M. J. Buchmeier. 2007. Mapping the landscape of the lymphocytic choriomeningitis virus stable signal peptide reveals novel functional domains. J. Virol. 81:5649-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi, Y., R. D. Beger, and J. M. Berg. 1993. Metal binding properties of single amino acid deletion mutants of zinc finger peptides: studies using cobalt(II) as a spectroscopic probe. Biophys. J. 64:749-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 57.South, T. L., P. R. Blake, R. C. Sowder III, L. O. Arthur, L. E. Henderson, and M. F. Summers. 1990. The nucleocapsid protein isolated from HIV-1 particles binds zinc and forms retroviral-type zinc fingers. Biochemistry 29:7786-7789. [DOI] [PubMed] [Google Scholar]
- 58.Spiropoulou, C. F., S. Kunz, P. E. Rollin, K. P. Campbell, and M. B. A. Oldstone. 2002. New World arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J. Virol. 76:5140-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strecker, T., R. Eichler, J. ter Meulen, W. Weissenhorn, H. D. Klenk, W. Garten, and O. Lenz. 2003. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particle. J. Virol. 77:10700-10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tam, R. C., J. Y. Lau, and Z. Hong. 2001. Mechanisms of action of ribavirin in antiviral therapies. Antivir. Chem. Chemother. 12:261-272. [DOI] [PubMed] [Google Scholar]
- 61.Tortorici, M. A., P. D. Ghiringhelli, M. E. Lozano, C. G. Albarino, and V. Romanowski. 2001. Zinc-binding properties of Junin virus nucleocapsid protein. J. Gen. Virol. 82:121-128. [DOI] [PubMed] [Google Scholar]
- 62.Tucker, P. A., D. Tsernoglou, A. D. Tucker, F. E. Coenjaerts, H. Leenders, and P. C. van der Vliet. 1994. Crystal structure of the adenovirus DNA binding protein reveals a hook-on model for cooperative DNA binding. EMBO J. 13:2994-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.York, J., S. S. Agnihothram, V. Romanowski, and J. H. Nunberg. 2005. Genetic analysis of heptad-repeat regions in the G2 fusion subunit of the Junin arenavirus envelope glycoprotein. Virology 343:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.York, J., and J. H. Nunberg. 2007. Distinct requirements for signal peptidase processing and function of the stable signal peptide (SSP) subunit in the Junin virus envelope glycoprotein. Virology 359:72-81. [DOI] [PubMed] [Google Scholar]
- 65.York, J., and J. H. Nunberg. 2006. Role of the stable signal peptide of the Junín arenavirus envelope glycoprotein in pH-dependent membrane fusion. J. Virol. 80:7775-7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.York, J., V. Romanowski, M. Lu, and J. H. Nunberg. 2004. The signal peptide of the Junín arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex. J. Virol. 78:10783-10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng, R., T. M. Jenkins, and R. Craigie. 1996. Zinc folds the N-terminal domain of HIV-1 integrase, promotes multimerization, and enhances catalytic activity. Proc. Natl. Acad. Sci. USA 93:13659-13664. [DOI] [PMC free article] [PubMed] [Google Scholar]