Abstract
A major factor in removing RNA primers during the processing of Okazaki fragments is DNA polymerase I (Pol I). Pol I is thought to remove the RNA primers and to fill the resulting gaps simultaneously. RNase H, encoded by rnh genes, is another factor in removing the RNA primers, and there is disagreement with respect to the essentiality of both the polA and rnh genes. In a previous study, we looked for the synthetic lethality of paralogs in Bacillus subtilis and detected several essential doublet paralogs, including the polA ypcP pair. YpcP consists of only the 5′-3′ exonuclease domain. In the current study, we first confirmed that the polA genes of both Escherichia coli and B. subtilis could be completely deleted. We found that the 5′-3′ exonuclease activity encoded by either polA or ypcP xni was required for the growth of B. subtilis and E. coli. Also, the 5′-3′ exonuclease activity of Pol I was indispensable in the cyanobacterium Synechococcus elongatus. These results suggest that a 5′-3′ exonuclease activity is essential in these organisms. Our success in constructing a B. subtilis strain that lacked all RNase H genes indicates that the enzymatic activity is dispensable, at least in the wild type. Increasing the 5′-3′ exonuclease activity partially compensated for a defective phenotype of an RNase H-deficient mutant, suggesting cooperative functions for the two enzyme systems. Our search for the distribution of the 5′-3′ exonuclease domain among 250 bacterial genomes resulted in the finding that all eubacteria, but not archaea, possess this domain.
Structural characterization and biochemical studies of several prokaryotic DNA polymerase I (Pol I, or PolA) established an organization in three functional domains: an N-terminal domain associated with a 5′-3′ exonuclease activity, a central domain that mediates proofreading of the 3′-5′ exonuclease activity, and a C-terminal domain responsible for the polymerase activity (33). In combination with two activities derived from both terminal domains, the so-called nick translation activity, Pol I has been thought to act simultaneously in removing RNA primers and in filling the resulting gaps. Evidently, the Escherichia coli polA1 mutant (7), which lacks polymerase activity but has 5′-3′ exonuclease activity, was able to grow, although it accumulated many Okazaki fragments, probably due to its inability to fill gaps (32). In addition, the polA(Ex1) mutant, which lacks 5′-3′ exonuclease activity at 43°C, also accumulated Okazaki fragments and could not grow at high temperatures (23).
RNase H specifically cleaves the RNA strand of RNA/DNA hybrids and plays a role in removing RNA primers of Okazaki fragments (29), although it cannot process a few ribonucleotides from the DNA-RNA junction sites. In fact, the isolation of a double mutant of polA and rnh (which encodes RNase H) made it possible to detect RNA primers and contributed to the determination of the RNA primer length as 9 to 12 nucleotides (20). Later progress revealed that the enzymes from a variety of prokaryotic and eukaryotic organisms could be classified into two major families, type 1 and type 2 RNase H (31). E. coli RNase HI, encoded by rnhA (17), belongs to the type 1 enzymes; E. coli RNase HII, encoded by rnhB (14), and Bacillus subtilis RNase HII (rnhB) and HIII (rnhC) (30) are categorized as type 2 enzymes. Biochemical characterization of several enzymes suggested that type 1 and type 2 RNase H are functionally related.
Previous studies disagreed regarding the essentiality of both the polA and rnh genes. The polA(Ex1) mutant described above exhibited temperature-sensitive growth, indicating a requirement for the 5′-3′ exonuclease catalytic domain of Pol I. Moreover, the polymerase or the 5′-3′ exonuclease domain was required for growth in rich media (16). On the other hand, as part of E. coli genome analysis, a complete knockout strain of polA that grew normally on LB medium was obtained, revealing the dispensability of polA (28). However, there have been no descriptions of the molecular mechanism(s) involved in the processing of RNA primers in polA mutants, although many studies involving work on the polA rnh double mutant described how they could support the initiation of replication of plasmids or chromosomes. Together, both the rnhA and rnhB genes were dispensable in E. coli in the presence of polA; on the other hand, the rnhB and rnhC genes of B. subtilis manifested synthetic lethality (15).
A number of microbial-genome-sequencing projects revealed that most bacterial genomes consist of about half singlet genes and half paralogous genes (24). Our comprehensive study, in which we looked for synthetic lethality of paralogs in B. subtilis, detected several essential functions of doublet paralogs (Y. Shiwa, S. Fukushima, and H. Yoshikawa, unpublished data); one of these was a polA ypcP pair. The YpcP protein is categorized as a 5′-3′ exonuclease (http://bacillus.genome.jp/); we considered this in our attempt to resolve the discrepancy that polA is dispensable even though 5′-3′ exonuclease activity is an essential function in the maturation of Okazaki fragments. The essentiality of the 5′-3′ exonuclease function in B. subtilis has been suggested by Duigou et al. (9), who reported less efficient transformation of a ΔpolA strain with a partially deleted ypcP gene of pMUTIN disruptant DNA. In the detailed analysis reported here, we used complete disruptants of entire genes or of domains of genes and an inducible gene expression system.
In addition to the two rnh genes described above, there are two more genes that code for RNase H in the B. subtilis genome. One of these, ypdQ, is similar in sequence to type 1 enzyme genes, although it has been reported that it lack critical amino acids in the active-site residues and that the purified protein exhibits no RNase H activity (30). To our surprise, an additional RNase H gene, ypeP, was found in the B. subtilis genome by reassignment of its open reading frame (S. Ishikawa, personal communication). In the current study, we first confirmed that the polA genes of both E. coli and B. subtilis could be completely deleted. Then, we demonstrated that a 5′-3′ exonuclease activity was indispensable in B. subtilis and in E. coli and is also in one of the cyanobacteria, Synechococcus elongatus strain PCC 7942, suggesting an essential function common to all organisms. Finally, we constructed a B. subtilis strain depleted of all RNase H genes. Our findings indicate that the RNase H enzymatic activity is dispensable, at least in the wild type. Based on these and other findings, we discuss the cooperation between 5′-3′ exonuclease and RNase H functions in vivo.
MATERIALS AND METHODS
Bacterial strains, plasmids, and genetic technique.
The bacterial strains used in this study are listed in Table 1. B. subtilis strains were maintained on Schaeffer's sporulation medium (37) and transformed with chromosome or plasmid DNA according to the method of Anagnostopoulos and Spizizen (1). Selection, when required, was done on erythromycin (1 μg/ml); on kanamycin, chloramphenicol, or neomycin (5 μg/ml); on tetracycline (15 μg/ml); and on spectinomycin (100 μg/ml). When necessary, the genes were overexpressed by cloning them in the multicopy plasmid pDG148 (40) or its derivative, pAN18, which contains multiple cloning sites of pUC18 in the HindIII site of pDG148 and is induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). The lacZ expression experiment was performed by growing B. subtilis cells in 2× SG medium (2) at 37°C, and aliquots of the culture were subjected to β-galactosidase activity assay as previously described (2).
TABLE 1.
Genotype of bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype | Source |
|---|---|---|
| B. subtilis strains | ||
| 168 | trpC2 | Laboratory stock |
| NBS5004 | trpC2 polA::spc | This study |
| NBS182 | trpC2 polA::erm | This study, |
| POLAd | trpC2 pMUTIN-polA erm | 22 |
| NBS5014 | trpC2 pMUTIN-T3CC-ypcP erm | This study |
| NBS5015 | trpC2 polA::spc pMUTIN-T3CC-ypcP erm | This study, NBS5014→ NBS5004 |
| NBS4070 | trpC2 ypcP::spc | This study |
| NBS183 | trpC2 polAC::cat | This study |
| NBS184 | trpC2 polAN::spc | This study |
| NBS185 | trpC2 polAC::cat ypcP::spc | This study, NBS183→ NBS4070 |
| NBS202 | trpC2 rnhB::spc | This study |
| BEST218 1A1plus | rnhB21::neo | 15 |
| NBS203 | trpC2 rnhB21::neo | This study, BEST218 1A1 plus→168 |
| NBS204 | trpC2 rnhC::cat | This study |
| NBS205 | trpC2 ypdQ::spc | This study |
| NBS206 | trpC2 ypeP::tet | This study |
| NBS207 | trpC2 rnhB::spc rnhC::cat | This study, NBS204→ NBS202 |
| NBS208 | trpC2 rnhB::spc ypeP::tet | This study, NBS206→ NBS202 |
| NBS209 | trpC2 rnhC::cat ypeP::tet | This study, NBS204→ NBS206 |
| NBS210 | trpC2 rnhB::spc rnhC::cat ypeP::tet | This study, NBS204→ NBS208 |
| NBS216 | trpC2 rnhB21::neo rnhC::cat ypeP::tet ypdQ::spc | This study, NBS204→ NBS224 |
| NBS217 | trpC2 rnhB21::neo rnhC::cat ypeP::tet ypdQ::spc polA::erm | This study, NBS182→ NBS216 |
| NBS218 | trpC2 rnhB::spc rnhC::cat ypeP::tet pMUTIN-yneA erm | This study, YNEAd→ NBS210 |
| NBS223 | trpC2 rnhB21::neo ypdQ::spc | This study, NBS205→ NBS203 |
| NBS224 | trpC2 rnhB21::neo ypeP::tet ypdQ::spc | This study, NBS206→ NBS223 |
| YNEAd | trpC2 pMUTIN-yneA erm | 22 |
| NBS247 | trpC2 polA::erm amyE′::pDR111a-polA spc::′amyE | This study, pDR111a-polA→NBS182 |
| NBS248 | trpC2 polA::erm amyE′::pDR111a-polA-Nspc::′amyE | This study, pDR111a-polAN→NBS182 |
| NBS249 | trpC2 polA::erm amyE′::pDR111a-ypcP spc::′amyE | This study, pDR111a-ypcP→NBS182 |
| E. coli strains | ||
| KM22 | argE3 his-4 leuB6 proA2 thr-1 ara-14 galK2 lacY1 mtl-1 xyl-5 thi-1 rpsL31 tsx-33 supE44 Δ(recC ptr recB recD)::Plac-bet exo kan | 27 |
| NBS198 | polA::cat | This study |
| NBS200 | xni::tet | This study |
| S. elongatus PCC 7942 strains | ||
| NBC100 | Wild type | Laboratory stock |
| NBC318 | ΔpolAC::kan | This study |
| Plasmids | ||
| pDG148 | bla kan | 40 |
| pAN18 | bla kan | This study |
| pMUTIN-T3CC | bla erm | Kei Asai |
| pDR111a | bla spc | Masaya Fujita |
E. coli K-12 strain KM22, derived from AB1157 (27), was grown on LB medium supplemented with 25 μg/ml kanamycin, and when necessary, 20 μg/ml chloramphenicol or 25 μg/ml tetracycline was added. Transformation was carried out as described previously (27).
The cyanobacterium S. elongatus PCC 7942 was grown in BG-11 (6) supplemented, when necessary, with 10 μg/ml kanamycin. Transformation of Synechococcus was done according to the method of Porter (34). We designed primer sequences based on information contained in the database of the very closely related strain S. elongatus PCC 6301 (http://cyano.genome.jp/).
Construction of strains carrying various deletion mutations.
Gene disruptant strains of B. subtilis and S. elongatus PCC 7942 were constructed via homologous recombination of PCR-generated fragments. The primary PCR-generated fragments contained around 600 to 1,000 bp of the upstream (using primers −11 and −12) (see Table S1 in the supplemental material) and downstream (primers −23 and −24) sequences of the target gene, both of which overlap either end of the PCR-generated fragment containing the antibiotic gene marker (primers -For and -Rev). In the case of E. coli, strain KM22 was used as the host and disruptants were obtained via homologous recombination provided by λ red function (27) with PCR-generated fragments. Fragments containing antibiotic resistance gene markers were amplified with a pair of 5′ and 3′ primers to which 40 bases upstream and downstream of the target gene were attached (see Table S1 in the supplemental material). The PCR products were directly used to transform KM22. The templates for the antibiotic markers in B. subtilis were Tn554 of Staphylococcus aureus for spectinomycin (spc) resistance; pC194 of S. aureus for chloramphenicol resistance (cat); pMUTIN-T3CC (K. Asai, unpublished data), a derivative of pMUTIN4 (42) for erythromycin (erm) resistance; and pBEST309 (13) of Enterococcus faecalis for tetracycline (tet) resistance. The templates for markers in E. coli were R100 plasmid for tetracycline (tet) resistance and the synthetic plasmid pHSG399 for chloramphenicol (cat) resistance. Plasmid pUC4K (Pharmacia) was used to confer kanamycin (kan) resistance on Synechococcus. To generate the desired PCR construct, we used the recombinant PCR method (12), in which the secondary-stage PCR products are generated with three-piece primary PCR fragments. All the constructs of gene disruptions were verified by diagnostic PCR assay. The PCR primers we used are listed in Table S1 in the supplemental material. Pyrobest DNA polymerase or ExTaq DNA polymerase (TaKaRa, Shiga, Japan) was used for PCR.
Construction of strains with an inducible expression system.
The B. subtilis strain harboring ypcP under the control of an IPTG-inducible promoter was constructed as follows. A 321-bp region containing the ribosomal binding sequences and the 5′ terminus of ypcP was amplified by PCR with a primer pair (see Table S1 in the supplemental material) and, after digestion with HindIII and BamHI, cloned into the HindIII/BamHI sites of pMUTIN-T3CC. The resulting plasmid was used to transform B. subtilis strain 168 to erythromycin resistance to obtain strain NBS5014. Consequently, in this integrant, ypcP was placed under the control of the IPTG-inducible spac promoter, and the promoter activity of ypcP itself could be monitored by its β-galactosidase activity (41). Alternatively, the B. subtilis strain harboring polA, the N-terminal region of polA, or ypcP under the control of the IPTG-inducible promoter Phyper-spank (derived from pDR111a) at the amyE locus in the ΔpolA mutant, was constructed. The fragments containing the full-length polA, the 5′-3′ exonuclease domain of polA, and ypcP were amplified by PCR with the same primer pairs cloned in pDG148 and, after digestion with XhoI or SalI and HindIII, cloned into the SalI/HindIII sites of pDR111a (gift from M. Fujita, University of Houston, Houston, TX) (5, 10). The resulting plasmids were used to transform B. subtilis strain NBS182 to obtain strain NBS247-249.
SOS induction assay.
As an indicator of SOS induction, the expression of yneA was analyzed with the transcriptionally fused lacZ reporter assay. In control experiments, strain YNEAd was grown to an optical density at 600 nm (OD600) of 0.35 in LB medium at 37°C and then treated with or without 50 ng/ml mitomycin C for 10 min. The mutagen-treated cells were harvested and resuspended in fresh medium, and culture was continued. At the indicated times, aliquots were withdrawn and β-galactosidase activity was measured. Simultaneously, NBS218 was grown in the absence of mitomycin C; when the culture reached the same OD600 as the withdrawn YNEAd, aliquots were assayed for β-galactosidase activity.
Bioinformatics.
All of the genome sequences were obtained from GenomeNet (http://www.genome.ad.jp/); domain structures were predicted by the SMART program (http://smart.embl-heidelberg.de/; 25, 38). The hidden Markov model (HMM) profiles of 3_5_exonuclease (PF01612) and DNA_pol_A (PF00476) were obtained from Pfam (http://www.sanger.ac.uk/Software/Pfam/). We built a custom profile HMM of 5_3_exonuclease based on the multiple sequence alignment of the 5′-3′ exonuclease domain generated by the SMART program (SMART accession no. SM00475). Motif searches for whole genomes were performed using the HMMER program; the e-value cutoff was 0.01.
RESULTS
The 5′-3′ exonuclease domain of either polA or ypcP is essential for B. subtilis.
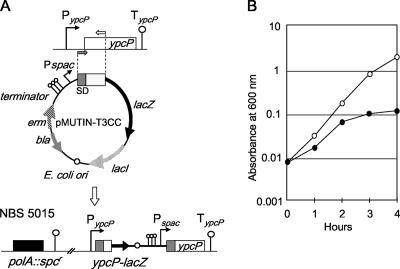
In the genome of B. subtilis, ypcP was found as a paralog of polA; our initial attempts to obtain a double-knockout mutant of these two genes failed. Therefore, we constructed the polA null mutant strain NBS5015, in which ypcP was placed under the IPTG-inducible promoter, as shown in Fig. 1A. NBS5015 can grow only in medium containing IPTG; Fig. 1B shows the growth defect after IPTG depletion.
FIG. 1.
Growth defect of the polA ypcP double mutant. (A) Scheme showing the gene structures of the polA and ypcP loci of NBS5015. (B) Growth profiles of strain NBS5015. NBS5015 was streaked on LB plates containing 0.01 mM IPTG and incubated overnight at 37°C. LB medium with (open circles) and without (closed circles) 1 mM IPTG was inoculated with diluted aliquots of the overnight culture (starting OD600 = 0.008) and incubated at 37°C. Growth was monitored by OD measurements.
Although ypcP was identified as a polA paralog, it encoded a small protein that corresponded only to the 5′-3′ exonuclease domain of PolA; this enzymatic activity appeared to be required for viability. To verify this, we tried to delete the C-terminal domains of PolA (designated ΔpolAC) in a ΔypcP background. We succeeded in constructing strain NBS185. Our results indicated that either ypcP or the 5′-3′ exonuclease domain of PolA sufficed for cell survival.
Characterization of polA and ypcP mutants and their expression profiles.
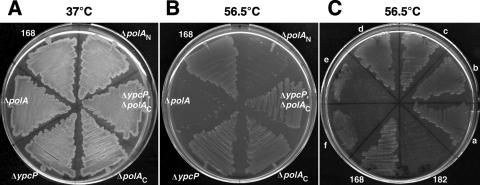
The E. coli polA null mutant manifests temperature-sensitive growth (16). We analyzed the growth profiles of various B. subtilis polA mutants in combination with the ypcP mutant. As shown in Fig. 2B, the entire polA disruptant (ΔpolA) also exhibited temperature-sensitive growth at 56.5°C in B. subtilis. On the other hand, the ΔypcP mutant was able to grow at this temperature, as was the wild type. Interestingly, the polA mutant (ΔpolAN) that lacked only the N-terminal 5′-3′ exonuclease domain was also temperature sensitive (Fig. 2B, NBS184); this was not true of the mutant lacking a Klenow fragment (ΔpolAC; NBS183). Moreover, the growth phenotype of the strain that had both ΔpolAC and ΔypcP was the same as that of the wild type. Together, these results demonstrated that the 5′-3′ exonuclease domain of polA was necessary for cell growth at high temperatures.
FIG. 2.
Growth of various polA and ypcP mutants on LB plates at 37°C (A) or 56.5°C (B). Relevant genotypes are shown: ΔpolA, polA deletion strain NBS5004; ΔypcP, ypcP deletion strain NBS4070; ΔpolAC, polA Klenow fragment deletion strain NBS183; ΔpolAN, polA 5′-3′ exonuclease domain deletion strain NBS184; ΔypcP, ΔpolAC, Klenow fragment and ypcP deletion strain NBS185. (C) Alternatively, the polA deletion strain NBS182 and its derivatives were examined on LB plates containing 1 mM IPTG at 56.5°C. Two independent transformants (each) of NBS247 (amyE::polA) (a and b), NBS248 (amyE::polAN) (c and d), and NBS249 (amyE::ypcP) (e and f) are shown.
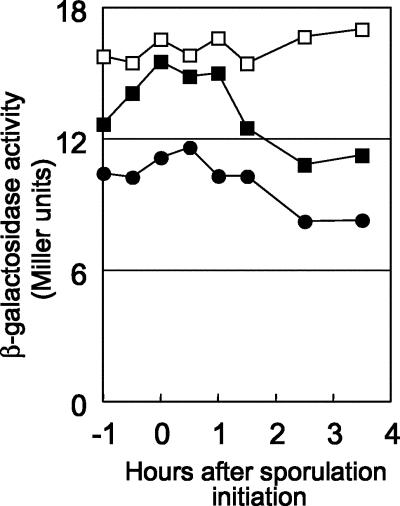
The ypcP gene encodes 296 amino acid residues that show 70% amino acid sequence homology with the 5′-3′ exonuclease domain of Pol I. As described above, lack of the polA 5′-terminal region gave rise to temperature-sensitive cell growth, while ΔypcP did not, suggesting that the natures of these two enzymes were different. As shown in Fig. 3, the expression profiles of the two genes were similar. Note that the expression level of ypcP-lacZ in the ΔpolA background was slightly higher than in the wild type; this may reflect compensation for the lack of 5′-3′ exonuclease activity. Consequently, we constructed the polA- or ypcP-inducible strain at the amyE locus. As shown in Fig. 2C, in the ΔpolA background (NBS182), strains harboring the polA N-terminal fragment suppressed the temperature sensitivity (Fig. 2C, c and d), as did control strains carrying full-length polA (Fig. 2C, a and b). On the other hand, ypcP-expressing strains (Fig. 2C, e and f) were unable to suppress the phenotype of the ΔpolA mutant completely. Note that the ypcP-expressing strain only partially suppressed temperature sensitivity at this temperature (Fig. 2C, e and f). These results confirmed that the temperature sensitivity was due to the lack of the 5′-3′ exonuclease domain of polA and that the two enzymes differed slightly in nature. As described below, multicopy ypcP, as well as polA, partially suppressed the RNase H deficiency; thus, these two enzymes share some functions, although their natures differ somewhat.
FIG. 3.
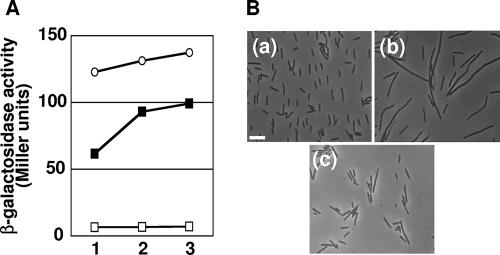
Expression profiles of β-galactosidase from polA-lacZ and ypcP-lacZ fusions. Each strain carrying lacZ fusions was grown in 2× SG medium, and β-galactosidase activity was assayed. The time of transition from exponential growth to stationary phase was designated time zero. Assays carried out more than three times yielded similar patterns; representative data are shown. Closed circles, POLAd (polA-lacZ); closed squares, NBS5014 (ypcP-lacZ); open squares, NBS5015 (ypcP-lacZ in the ΔpolA background).
Another function of Pol I is as a repair enzyme in the SOS response. Presumably, this function is derived from the Klenow fragment of Pol I and YpcP is not involved. As indicated in Table 2, the ΔpolA strain showed profound sensitivity to the DNA-damaging agent mitomycin C; the reaction of the ΔypcP strain was comparable to that of the wild type. In addition, the expression of neither of the genes was induced by mitomycin C treatment (data not shown). This is in contrast to E. coli polA (43). It should be noted that a global mobility shift assay and DNA microarray analysis identified no LexA binding site in the upstream region of B. subtilis polA (3).
TABLE 2.
Test of the sensitivity of B. subtilis strains to mitomycin Ca
| Strain | Relevant genotype | Surviving fraction |
|---|---|---|
| 168 | Wild type | 0.54 |
| NBS5004 | ΔpolA | 2.2 × 10−8 |
| NBS4070 | ΔypcP | 0.27 |
Cells were grown exponentially (optical density at 600 nm, 0.4) at 37°C in LB medium supplemented with appropriate antibiotics. Mitomycin C sensitivity was tested by plating serial dilutions of the culture onto LB and LB supplemented with 50 ng/ml mitomycin C.
xni is an E. coli counterpart of ypcP.
The E. coli ortholog of ypcP is xni; it has been reported to encode an exonuclease (http://genolist.pasteur.fr/Colibri/). Based on our results with B. subtilis, we tested whether the synthetic lethality of polA and xni applied to E. coli. First, we constructed a polA and xni disruptant of E. coli KM22 independently. Then, we transformed the xni disruptant with ΔpolA chromosomal DNA to obtain a double-knockout strain; however, this effort failed. The phenotype of the polA disruptant we constructed was similar to that reported by Joyce and Grindley (16) in that neither grew on LB medium or could be rendered competent because of their slow growth in minimal medium. No distinct transformant was obtained, although control experiments in transformation of the wild-type strain using the same DNA yielded more than a hundred transformants. For example, when we used a fragment containing a polA::cat cassette to transform KM22, we obtained approximately 100 transformants at 1 μg DNA. On the other hand, when we used the same DNA fragment to transform NBS200 (xni::tet), the few colonies that appeared were confirmed to be disrupted in both the polA and xni genes. However, the recipient cells yielded a normal number (>100) of transformants with other genetic markers. Therefore, we postulated that these transformants were suppressors and concluded that polA and xni exhibited synthetic lethality. Our results indicated that 5′-3′ exonuclease activities encoded by either polA or xni were also essential in E. coli.
The N-terminal domain of cyanobacterial polA is indispensable.
The genome of the cyanobacterium S. elongatus PCC 7942 does not encode a YpcP ortholog. Therefore, we examined the essentiality of polA (Synpcc7942_0194). As described in Materials and Methods, we attempted to construct the entire polA gene knockout strain with the 7942polA series primer and the C-terminal Klenow domain disruptant using the 7942polAc series primer (see Table S1 in the supplemental material). Since cyanobacteria contain multiple copies of the chromosome, it is usually possible to obtain disruptant colonies for the essential gene. However, after several successive patchings, only nonessential genes could be completely replaced. Using this criterion, the C-terminal disruptant was stably maintained, and PCR analysis confirmed complete replacement in the genome with the mutant allele. The resulting strain was named NBC318. On the other hand, the entire polA gene disruptant could not be maintained stably, and PCR analysis of these colonies revealed that the wild-type allele of polA was retained (data not shown). These results demonstrated that the 5′-3′ exonuclease domain of Synechococcus Pol I is essential for growth.
RNase H function is dispensable in B. subtilis.
RNase H, encoded by rnh genes, is another factor in removing the RNA primers, although it cannot process a few ribonucleotides at the DNA-RNA junction. Four genes encoding RNase H in the B. subtilis genome, i.e., rnhB, rnhC, ypdQ, and ypeP, have been identified. YpeP, which is similar to RNase HI, was newly identified; it possessed all known conserved residues involved in the catalysis of E. coli RnhA, i.e., D10, E48, D70, H124, and D134 (Fig. 4). Each RNase H gene disruptant exhibited a normal growth phenotype, although rnhC and ypeP mutants formed only small colonies, even on complete medium. It has been reported that both the rnhB and rnhC genes confer synthetic lethality on the cells and that in B. subtilis, unlike in E. coli, RNase H is indispensable (15). However, we obtained a B. subtilis strain that lacked both rnhB and rnhC, although the colony size was rather small and the cell morphology was filamentous. Moreover, disruptants in three and all four RNase H genes were able to grow at 37°C (Table 3). Mutants lacking both rnhB and rnhC showed temperature-sensitive growth at 56.5°C, and strain NBS210, which additionally lacked ypeP, exhibited cold-sensitive growth at 22°C. The growth rates in LB medium of these high- or low-temperature-sensitive mutants at 37°C were about half of that of the wild type (data not shown).
FIG. 4.
Multiple alignments of type 1 RNase H sequences. The B. subtilis RNase HI homolog YpdQ and the novel RNase HI candidate YpeP were aligned with E. coli RNase HI (eRnhA) and Halobacterium RNase H (hRnh). The asterisks indicate the conserved amino acid residues with more than 65% identity.
TABLE 3.
Cold-sensitive or temperature-sensitive phenotypes of various gene disruption strains
| Strain | Relevant genotype | Growth ata:
|
||
|---|---|---|---|---|
| 22°C | 37°C | 56.5°C | ||
| 168 | Wild type | + | + | + |
| NBS5004 | ΔpolA | + | + | − |
| NBS4070 | ΔypcP | + | + | + |
| NBS202 | ΔrnhB | + | + | + |
| NBS204 | ΔrnhC | + | + | + |
| NBS205 | ΔypdQ | + | + | + |
| NBS206 | ΔypeP | + | + | + |
| NBS208 | ΔrnhB ΔypeP | + | + | + |
| NBS209 | ΔrnhC ΔypeP | + | + | + |
| NBS207 | ΔrnhB ΔrnhC | + | + | − |
| NBS210 | ΔrnhB ΔrnhC ΔypeP | − | + | − |
| NBS216 | ΔrnhB ΔrnhC ΔypeP ΔypdQ | − | + | − |
| NBS217 | ΔpolA ΔrnhB ΔrnhC ΔypeP ΔypdQ | − | + | − |
+, growth; −, no growth.
The filamentous phenotype of RNase H-defective strains is due to an SOS response.
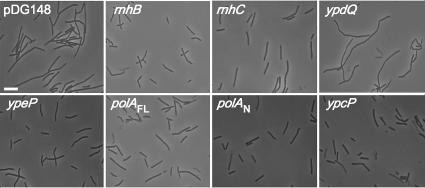
When the triple mutant NBS210 was grown in LB medium at 37°C, many of the cells at mid-log phase exhibited filamentous cell morphology (Fig. 5, pDG148). This filamentous phenotype was suppressed when any one of the depleted genes, rnhB, rnhC, or ypeP, was propagated in a multicopy plasmid. On the other hand, overexpression of ypdQ had no effect on the morphology of NBS210 (Fig. 5).
FIG. 5.
Effects of overexpression of various genes on the filamentous phenotype of a B. subtilis triple-RNase H-deficient strain. NBS210 harboring pDG148 or its derivatives was grown at 37°C in LB medium containing 5 μg/ml kanamycin and 1 mM IPTG. Cell morphologies at mid-log phase were observed under a microscope. Genes cloned in pDG148 are shown. FL and N indicate full-length and N-terminal domains. Bar, 10 μm.
RNase H deficiency is thought to result in the accumulation of Okazaki fragments and may induce an SOS response. To examine this possibility, we performed a lacZ reporter assay of yneA. The expression of yneA is under the control of LexA and is induced by an SOS response whose functions overlap those of SulA in E. coli (18). As shown in Fig. 6A, yneA-lacZ is constitutively expressed in NBS218, an NBS210 derivative carrying a yneA-lacZ construct. The level of expression was remarkably higher than in the wild type, even when a cellular SOS response was induced by mitomycin C. Moreover, the yneA disruptant of NBS210 (NBS218) ceased to exhibit the filamentous phenotype, and consequently, the impairment of cell division exhibited by the RNase H-defective strain was suppressed (Fig. 6B).
FIG. 6.
SOS induction assay. (A) Kinetics of yneA-lacZ induction. Aliquots of the YNEAd culture treated with (closed squares) or without (open squares) mitomycin C were withdrawn 20 (lane 1), 40 (lane 2), and 60 (lane 3) min after drug treatment and assayed for β-galactosidase activity. Portions of an alternatively grown NBS218 culture, a triple-RNase H-deficient strain, were sampled (open circles) as described in Materials and Methods and assayed for β-galactosidase activity. (B) Effects of yneA deletion on B. subtilis cell morphology. Cells from exponentially growing cultures were examined under a microscope. (a) 168 (wild type). (b) Triple-RNase H-deficient strain NBS210. (c) Triple-RNase H- and yneA-deficient strain NBS218. Bar, 10 μm.
Partial complementation of RNase H by 5′-3′ exonuclease activity.
It has been suggested that both RNase H and Pol I function synergistically during the processing of RNA primers of Okazaki fragments (35). Hence, the deficiency of RNase H might be compensated for by enhanced 5′-3′ exonuclease activity. As shown in Fig. 5 (polAFL and ypcP), the filamentous phenotype of strain NBS210 was obviously suppressed when the polA or ypcP gene was overexpressed. A similar result was obtained for the gene encoding only the N-terminal domain of Pol I (Fig. 5, polAN). These results indicate that the filamentous morphology was due to defective RNase H activity and that overexpression of either of the 5′-3′ exonuclease domains could complement the rnh mutations.
DISCUSSION
Our discovery of the synthetic lethality of a pair of doublet paralogs, polA and ypcP, confirmed previous results (9) and more clearly demonstrated that not polymerase but 5′-3′ exonuclease activity is an essential function for B. subtilis cells. This and findings for E. coli and cyanobacteria suggest that either the 5′-3′ exonuclease activity of Pol I or the activity encoded by ypcP xni is required for bacterial growth.
With the assistance of RNase H, the excision of RNA primers in vitro was promoted (11). RNase H genes have been reported to be essential in B. subtilis (15). However, as we succeeded in disrupting all candidate genes for RNase H, we conclude that RNase H is not necessary for cell viability. However, the growth rates of strains bearing two or more RNase H gene deletions, including rnhB and rnhC, were low, suggesting that RNase H is involved in the processing of RNA primers.
Based on the colony morphologies of individual disruptants, rnhC appears to be the most prominent among the four RNase H genes; an rnhB rnhC double mutant exhibited temperature-sensitive growth and filamentous cell morphology. Moreover, these phenotypes were apparently the same for all four gene disruptants, suggesting that the major functions of RNase H are attributable to these two genes. The overexpression of the newly identified ypeP suppressed filamentation of the rnhB rnhC ypeP mutant, while ypdQ did not. Although some (21) have claimed that YpeP lacks RNase H activity based on structural requirements, the revised assignment of open reading frames and careful alignment revealed conservation of all active amino acids (Fig. 4). These results suggest that YpeP has some RNase H activity and that YpdQ does not and support earlier findings that ypdQ did not function in B. subtilis (15, 30).
Among the six genes related to the processing of RNA primers of Okazaki fragments, five could be disrupted, and ypcP alone could confer viability on cells. The expression profiles of ypcP and polA were similar. The strain lacking the N-terminal domain of Pol I (ΔpolAN) was temperature sensitive, and propagation of the ypcP gene in the amyE locus could not suppress the temperature sensitivity of the polA mutant (Fig. 2C), suggesting a difference in the natures of these two 5′-3′ exonucleases. It should be noted that the partial suppression observed in Fig. 2C may be due to the gene dosage effect of the presence of two copies of the ypcP gene in the genome. Hence, the activity of YpcP seems to be inactive at high temperature, although in vitro experiments demonstrated that it possesses 5′-3′ exonuclease activity (J. Sekiguchi, personal communication). Moreover, RNase H alone could not remove RNA primers completely (30), and the temperature-sensitive phenotype of the ΔpolA strain can thus be elucidated. Although the RNase H disruptant was also identified as temperature sensitive regardless of Pol I activity, we strongly suspect that RNase H function, in addition to Pol I, is required for the efficient processing of Okazaki fragments (35) under high-temperature conditions.
The expression profiles of yneA-lacZ in the multiple-gene disruptants, including rnhB and rnhC, clearly demonstrated that the SOS response had taken place. In E. coli, the SOS response leads to sulA expression and inhibits Z-ring formation (26). In B. subtilis, the detailed mechanism is different, but the enhanced activity of YneA indirectly inhibits Z-ring formation (18, 19). Hence, the filamentous cell morphology may be a consequence of depleted Z-ring formation attributable to the activation of the LexA repressor due to accumulated unprocessed Okazaki fragments. The basic regulatory mechanism of the SOS response is controlled by a complex circuitry involving RecA and LexA proteins. When chromosomal DNA is damaged or its replication is inhibited, RecA is activated by inducing signals and the LexA repressor is degraded. It has been reported that a polA mutant, as well as an RNase H mutant, accumulate Okazaki fragments and fail to survive (32). The relationship between SOS induction and temperature sensitivity remains unknown; however, our results in B. subtilis indicate that inefficient processing of RNA primers of the lagging strand results in recognition of DNA damage and leads to both SOS induction and temperature sensitivity.
In Haemophilus influenzae, the mutation of RNase HI increases the mutation rates of tetranucleotide repeats and results in phase variation due to delayed or mutagenic Okazaki fragment processing; the additional deletion of the Klenow domain of Pol I gives rise to much higher phase variation rates (4). These results also support the hypothesis that two enzyme systems cooperate in the processing of Okazaki fragments.
The dispensability of the RNase H function was attributed to complementation of the 5′-3′ exonuclease activities of Pol I and YpcP. The filamentous phenotype induced by deficient RNase H was partially suppressed by the overexpression of polA, the N-terminal part of polA, or ypcP. These results suggest that enhanced 5′-3′ exonuclease activity suppressed the RNase H deficiency, and therefore, the two enzymes appear to cooperate.
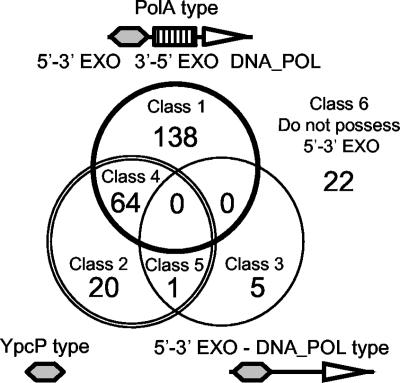
Our results also indicated that the 5′-3′ exonuclease activity was indispensable in E. coli, as well as in the cyanobacterial strain S. elongatus PCC 7942. It has been reported that the 5′-3′ exonuclease activity of Pol I appears to be essential in two other bacteria, H. influenzae and Streptococcus pneumoniae, which also lack the ypcP ortholog (4, 8). Hence, this enzymatic activity seems to be essential for all eubacterial cells. Our finding of B. subtilis ypcP and its E. coli ortholog, xni, led us to look for the distribution of two types of 5′-3′ exonucleases among bacteria. E. coli xni was first identified and designated exo (36), and the encoded enzyme was previously reported to have mainly 3′-5′ single-stranded DNA exonuclease activity and some 5′-3′ exonuclease activity (39). Although its characteristic nature in RNA/DNA hybrids has not been analyzed, we posit that it may process a substrate in the same manner as YpcP, and we classified the genes based on their structural similarities. We categorized genes possessing a 5′-3′ exonuclease domain into three types, i.e., polA type, ypcP type, and a third type, consisting of a 5′-3′ exonuclease domain and a polymerase domain in which the 3′-5′ exonuclease domain is less homologous to that of polA (e-value < 0.01). A total of 250 bacterial genomes so far sequenced were classified into six classes, as shown in Fig. 7 (see also Fig. S1 in the supplemental material). Both B. subtilis and E. coli belong to class 4, although the 3′-5′ exonuclease activity of B. subtilis is known to be absent (9). Archaea (class 6) are exceptional because, instead of 5′-3′ exonucleases, like eukaryotes, they have flap endonucleases. On the other hand, all eubacteria have at least one type of 5′-3′ exonuclease. About 90% of the eubacterial genomes contain polA-type genes; 37% carry ypcP-type genes. Only five genomes have type 3 genes (class 3). On the other hand, 20 genomes have ypcP-type genes (class 2) exclusively, indicating that neither the 3′-5′ exonuclease nor the polymerase domain is essential.
FIG. 7.
Venn diagram of bacterial species according to the type of 5′-3′ exonuclease gene. A total of 250 bacterial genomes (228 eubacteria and 22 archaea) sequenced to date were categorized into six classes based on the gene structure encoding 5′-3′ exonuclease. Each circle corresponds to three types of 5′-3′ exonuclease: PolA type, YpcP type, and a third (5′-3′ EXO-DNA_POL) type (see the text). Class 1 consisted of genomes containing only the PolA type. Class 2 consisted of genomes containing only the YpcP type. Class 3 consisted of genomes containing only the third type. Class 4 consisted of genomes containing the PolA type and the YpcP type. Class 5 consisted of genomes containing the YpcP type and the third type. Class 6 consisted of genomes containing no 5′-3′ exonuclease genes.
Therefore, we used the informatics approach to look for the distribution of repair-related proteins other than PolA, i.e., PolB and the IMS domain, which includes Pol IV and Pol V (see Fig. S1 in the supplemental material). Using this analysis, we found that all archaea possess DNA polymerase II and only seven species, which simultaneously belong to class 2 in Fig. 7, possess no repair polymerase (see Fig. S1 in the supplemental material). These species belong to four genera, Buchnera, Wigglesworthia, Phytoplasma, and Tropheryma, and all are symbiotic or infectious bacteria. Although it is not yet known whether these bacteria are sensitive to DNA-damaging agents, such as mitomycin C, they may not require DNA repair functions because of the environments they inhabit.
As noted above, archaea and eubacteria developed distinct types of processing enzymes, i.e., flap endonuclease and 5′-3′ exonuclease, respectively. There are weak homologies among the three domains of polymerase I, and the hyperthermophilic bacterium Aquifex aeolicus, which belongs to class 2, diverged close to the root of the phylogenetic tree. Since Aquifex also possesses a gene corresponding to a separate Klenow fragment, one of the three domains, i.e., 5′-3′ exonuclease, evolved first, and duplication and combination of this unit may have yielded a variety of related enzyme patterns among different organisms.
Supplementary Material
Acknowledgments
We are grateful to Naoto Ohtani of Keio University for helpful discussion on the alignment of RNase H and to Shinichi Matsuyama of Rikkyo University for providing the KM22 strain and for technical support in E. coli transformation. We thank Shu Ishikawa of the Nara Institute of Science and Technology for providing valuable information on the ypeP reassignment. We also thank Masaya Fujita for plasmids and Yukari Sato for technical assistance.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Area (C) (Genome Biology) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Published ahead of print on 28 September 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation In Bacillus subtilis. J. Bacteriol. 81:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai, K., F. Kawamura, A. Hirata, H. Yoshikawa, and H. Takahashi. 1993. SecA is required for three distinct stages of sporulation in Bacillus subtilis. J. Gen. Appl. Microbiol. 39:583-596. [Google Scholar]
- 3.Au, N., E. Kuester-Schoeck, V. Mandava, L. E. Bothwell, S. P. Canny, K. Chachu, S. A. Colavito, S. N. Fuller, E. S. Groban, L. A. Hensley, T. C. O'Brien, A. Shah, J. T. Tierney, L. L. Tomm, T. M. O'Gara, A. I. Goranov, A. D. Grossman, and C. M. Lovett. 2005. Genetic composition of the Bacillus subtilis SOS system. J. Bacteriol. 187:7655-7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayliss, C. D., W. A. Sweetman, and E. R. Moxon. 2005. Destabilization of tetranucleotide repeats in Haemophilus influenzae mutants lacking RnaseHI or the Klenow domain of PolI. Nucleic Acids Res. 33:400-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britton, R. A., P. Eichenberger, J. E. Gonzalez-Pastor, P. Fawcett, R. Monson, R. Losick, and A. D. Grossman. 2002. Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. J. Bacteriol. 184:4881-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castenholz, R. W. 1988. Culturing methods for cyanobacteria. Methods Enzymol. 167:68-93. [Google Scholar]
- 7.De Lucia, P., and J. Cairns. 1969. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature 224:1164-1166. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, A., S. A. Lacks, and P. Lopez. 1992. The 5′ to 3′ exonuclease activity of DNA polymerase I is essential for Streptococcus pneumoniae. Mol. Microbiol. 6:3009-3019. [DOI] [PubMed] [Google Scholar]
- 9.Duigou, S., S. D. Ehrlich, P. Noirot, and M. F. Noirot-Gros. 2005. DNA polymerase I acts in translesion synthesis mediated by the Y-polymerases in Bacillus subtilis. Mol. Microbiol. 57:678-690. [DOI] [PubMed] [Google Scholar]
- 10.Fujita, M., and R. Losick. 2005. Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A. Genes Dev. 19:2236-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funnell, B. E., T. A. Baker, and A. Kornberg. 1986. Complete enzymatic replication of plasmids containing the origin of the Escherichia coli chromosome. J. Biol. Chem. 261:5616-5624. [PubMed] [Google Scholar]
- 12.Higuchi, R. 1989. Using PCR to engineer DNA, p. 61-70. In H. A. Erlich (ed.), PCR technology. Stockton Press, New York, NY.
- 13.Itaya, M. 1992. Construction of a novel tetracycline resistance gene cassette useful as a marker on the Bacillus subtilis chromosome. Biosci. Biotechnol. Biochem. 56:685-686. [DOI] [PubMed] [Google Scholar]
- 14.Itaya, M. 1990. Isolation and characterization of a second RNase H (RNase HII) of Escherichia coli K-12 encoded by the rnhB gene. Proc. Natl. Acad. Sci. USA 87:8587-8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itaya, M., A. Omori, S. Kanaya, R. J. Crouch, T. Tanaka, and K. Kondo. 1999. Isolation of RNase H genes that are essential for growth of Bacillus subtilis 168. J. Bacteriol. 181:2118-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyce, C. M., and N. D. Grindley. 1984. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J. Bacteriol. 158:636-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanaya, S., and R. J. Crouch. 1983. DNA sequence of the gene coding for Escherichia coli ribonuclease H. J. Biol. Chem. 258:1276-1281. [PubMed] [Google Scholar]
- 18.Kawai, Y., S. Moriya, and N. Ogasawara. 2003. Identification of a protein, YneA, responsible for cell division suppression during the SOS response in Bacillus subtilis. Mol. Microbiol. 47:1113-1122. [DOI] [PubMed] [Google Scholar]
- 19.Kawai, Y., and N. Ogasawara. 2006. Bacillus subtilis EzrA and FtsL synergistically regulate FtsZ ring dynamics during cell division. Microbiology 152:1129-1141. [DOI] [PubMed] [Google Scholar]
- 20.Kitani, T., K. Yoda, T. Ogawa, and T. Okazaki. 1985. Evidence that discontinuous DNA replication in Escherichia coli is primed by approximately 10 to 12 residues of RNA starting with a purine. J. Mol. Biol. 184:45-52. [DOI] [PubMed] [Google Scholar]
- 21.Knizewski, L., and K. Ginalski. 2005. Bacillus subtilis YkuK protein is distantly related to RNase H. FEMS Microbiol. Lett. 251:341-346. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi, K., S. D. Ehrlich, A. Albertini, G. Amati, K. K. Andersen, M. Arnaud, K. Asai, S. Ashikaga, S. Aymerich, P. Bessieres, F. Boland, S. C. Brignell, S. Bron, K. Bunai, J. Chapuis, L. C. Christiansen, A. Danchin, M. Debarbouille, E. Dervyn, E. Deuerling, K. Devine, S. K. Devine, O. Dreesen, J. Errington, S. Fillinger, S. J. Foster, Y. Fujita, A. Galizzi, R. Gardan, C. Eschevins, T. Fukushima, K. Haga, C. R. Harwood, M. Hecker, D. Hosoya, M. F. Hullo, H. Kakeshita, D. Karamata, Y. Kasahara, F. Kawamura, K. Koga, P. Koski, R. Kuwana, D. Imamura, M. Ishimaru, S. Ishikawa, I. Ishio, D. Le Coq, A. Masson, C. Mauel, R. Meima, R. P. Mellado, A. Moir, S. Moriya, E. Nagakawa, H. Nanamiya, S. Nakai, P. Nygaard, M. Ogura, T. Ohanan, M. O'Reilly, M. O'Rourke, Z. Pragai, H. M. Pooley, G. Rapoport, J. P. Rawlins, L. A. Rivas, C. Rivolta, A. Sadaie, Y. Sadaie, M. Sarvas, T. Sato, H. H. Saxild, E. Scanlan, W. Schumann, J. F. Seegers, J. Sekiguchi, A. Sekowska, S. J. Seror, M. Simon, P. Stragier, R. Studer, H. Takamatsu, T. Tanaka, M. Takeuchi, H. B. Thomaides, V. Vagner, J. M. van Dijl, K. Watabe, A. Wipat, H. Yamamoto, M. Yamamoto, Y. Yamamoto, K. Yamane, K. Yata, K. Yoshida, H. Yoshikawa, U. Zuber, and N. Ogasawara. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 100:4678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornberg, A., and T. Baker. 1991. DNA replication. W. H. Freeman and Company, New York, NY.
- 24.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 25.Letunic, I., R. R. Copley, B. Pils, S. Pinkert, J. Schultz, and P. Bork. 2006. SMART 5: domains in the context of genomes and networks. Nucleic Acids Res. 34:D257-D260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee, A., C. Cao, and J. Lutkenhaus. 1998. Inhibition of FtsZ polymerization by SulA, an inhibitor of septation in Escherichia coli. Proc. Natl. Acad. Sci. USA 95:2885-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy, K. C. 1998. Use of bacteriophage lambda recombination functions to promote gene replacement in Escherichia coli. J. Bacteriol. 180:2063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata, Y., K. Mashimo, M. Kawata, and K. Yamamoto. 2002. The roles of Klenow processing and flap processing activities of DNA polymerase I in chromosome instability in Escherichia coli K12 strains. Genetics 160:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa, T., and T. Okazaki. 1984. Function of RNase H in DNA replication revealed by RNase H defective mutants of Escherichia coli. Mol. Gen. Genet. 193:231-237. [DOI] [PubMed] [Google Scholar]
- 30.Ohtani, N., M. Haruki, M. Morikawa, R. J. Crouch, M. Itaya, and S. Kanaya. 1999. Identification of the genes encoding Mn2+-dependent RNase HII and Mg2+-dependent RNase HIII from Bacillus subtilis: classification of RNases H into three families. Biochemistry 38:605-618. [DOI] [PubMed] [Google Scholar]
- 31.Ohtani, N., M. Haruki, M. Morikawa, and S. Kanaya. 1999. Molecular diversities of RNases H. J. Biosci. Bioeng 88:12-19. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki, T. 2002. Okazaki fragments and discontinuous replication. Seikagaku 74:103-117. [In Japanese.] [PubMed] [Google Scholar]
- 33.Patel, P. H., M. Suzuki, E. Adman, A. Shinkai, and L. A. Loeb. 2001. Prokaryotic DNA polymerase I: evolution, structure, and “base flipping” mechanism for nucleotide selection. J. Mol. Biol. 308:823-837. [DOI] [PubMed] [Google Scholar]
- 34.Porter, R. D. 1988. DNA transformation. Methods Enzymol. 167:703-712. [DOI] [PubMed] [Google Scholar]
- 35.Sato, A., A. Kanai, M. Itaya, and M. Tomita. 2003. Cooperative regulation for Okazaki fragment processing by RNase HII and FEN-1 purified from a hyperthermophilic archaeon, Pyrococcus furiosus. Biochem. Biophys. Res. Commun. 309:247-252. [DOI] [PubMed] [Google Scholar]
- 36.Sayers, J. R. 1994. Computer aided identification of a potential 5′-3′ exonuclease gene encoded by Escherichia coli. J. Theor. Biol 170:415-421. [DOI] [PubMed] [Google Scholar]
- 37.Schaeffer, P., J. Millet, and J. P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafritz, K. M., M. Sandigursky, and W. A. Franklin. 1998. Exonuclease IX of Escherichia coli. Nucleic Acids Res. 26:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stragier, P., C. Bonamy, and C. Karmazyn-Campelli. 1988. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell 52:697-704. [DOI] [PubMed] [Google Scholar]
- 41.Vagner, V., E. Dervyn, and S. D. Ehrlich. 2001. Inactivation of Bacillus subtilis genes without known functions, p. 21-24. In W. Schumann, S. D. Ehrlich, and N. Ogasawara (ed.), Functional analysis of bacterial genes. Wiley, Chichester, United Kingdom.
- 42.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 43.Wandt, G., S. Kubis, and A. Quinones. 1997. Treatment with DNA-damaging agents increases expression of polA′-′lacZ gene fusions in Escherichia coli K-12. Mol. Gen. Genet. 254:98-103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.