Abstract
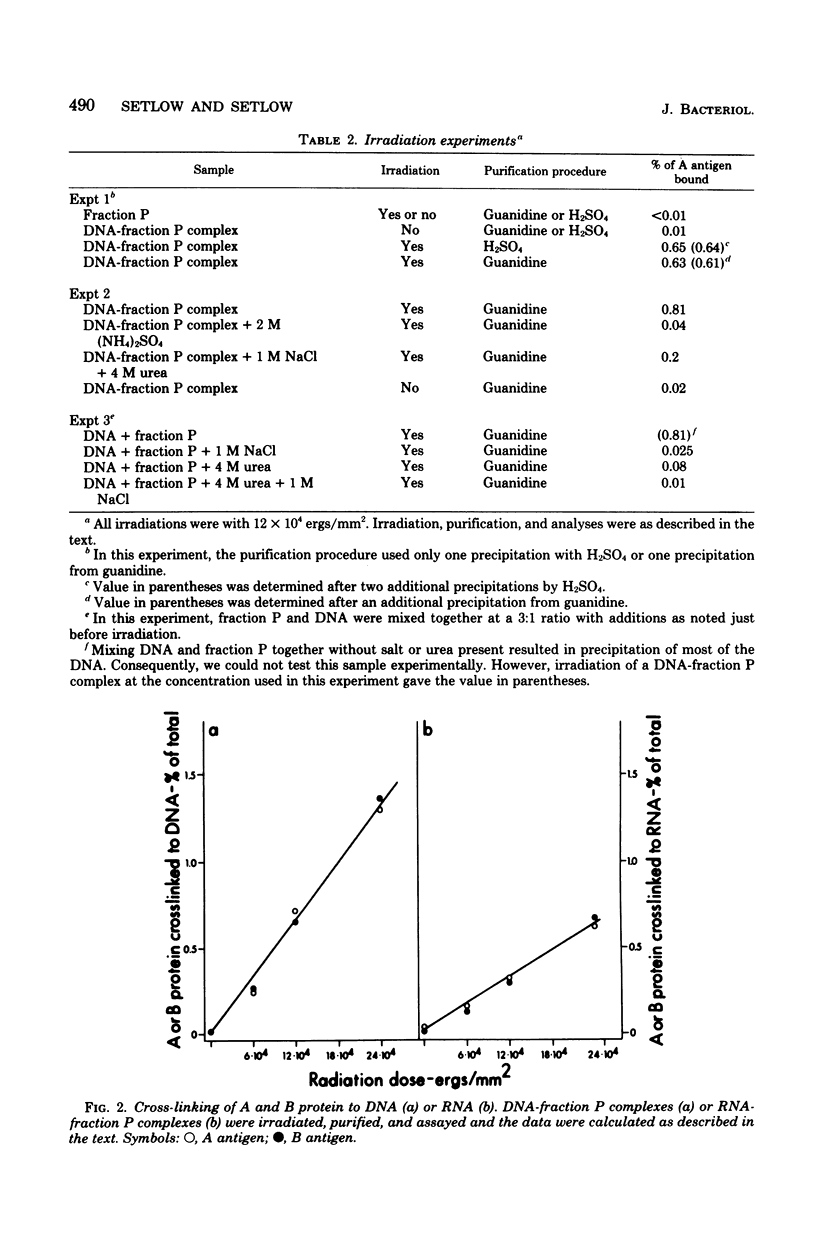
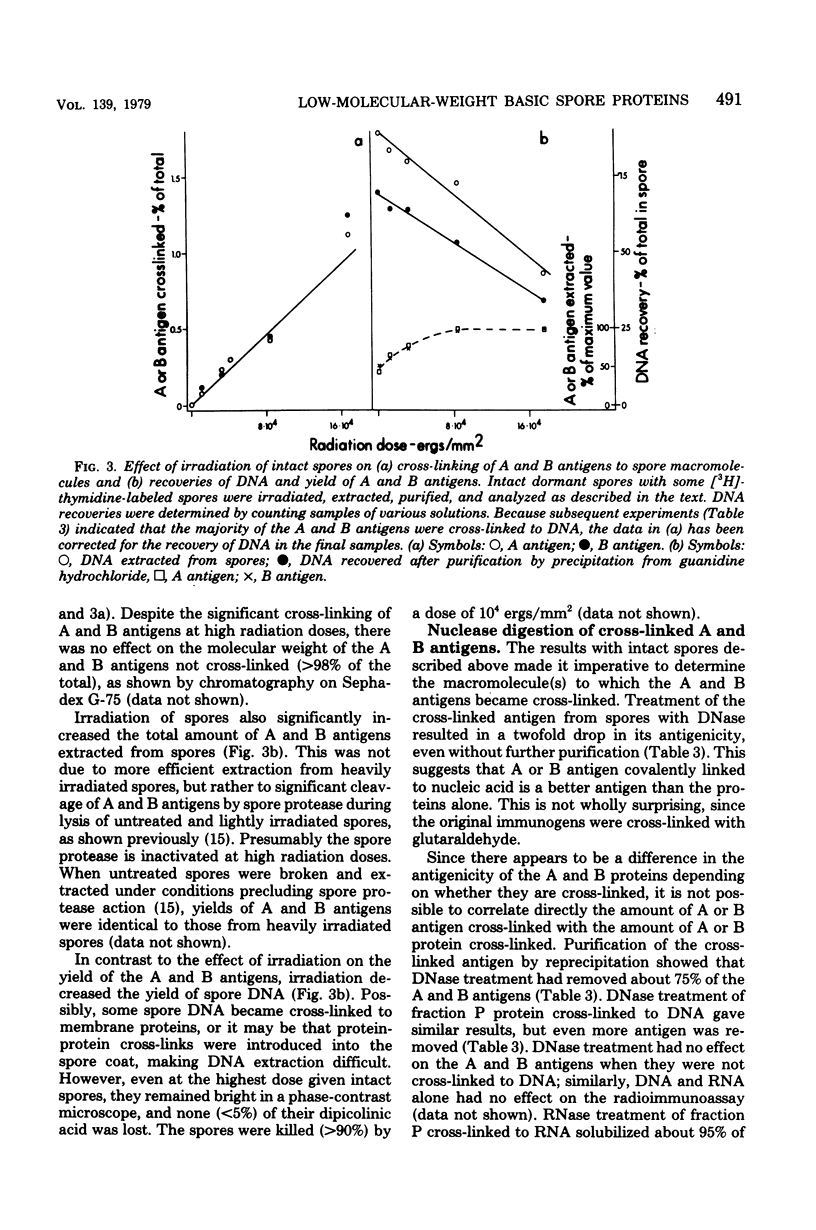
Two low-molecular-weight basic proteins, termed A and B proteins, comprise about 15% of the protein of dormant spores of Bacillus megaterium. Irradiation of intact dormant spores with ultraviolet light results in covalent cross-linking of the A and B proteins to other spore macromolecules. The cross-linked A and B proteins are precipitated by ethanol and can be solubilized by treatment with deoxyribonuclease (75%) or ribonuclease (25%). Irradiation of complexes formed in vitro between deoxyribonucleic acid (DNA) or ribonucleic acid and a mixture of the low-molecular-weight basic proteins from spores also resulted in cross-linking of A and B proteins to nucleic acids. The dose-response curves for formation of covalent cross-links were similar for irradiation of both a protein-DNA complex in vitro and intact spores. However, if irradiation was carried out in vitro under conditions where DNA-protein complexes were disrupted, no covalent cross-links were formed. These data suggest that significant amounts of the low-molecular-weight basic proteins unique to bacterial spores are associated with spore DNA in vivo.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baillie E., Germaine G. R., Murrell W. G., Ohye D. F. Photoreactivation, photoproduct formation, and deoxyribonucleic acid state in ultraviolet-irradiated sporulating cultures of Bacillus cereus. J Bacteriol. 1974 Oct;120(1):516–523. doi: 10.1128/jb.120.1.516-523.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen E. L., Marquis R. E., Gerhardt P. Dielectric study of the physical state of electrolytes and water within Bacillus cereus spores. J Bacteriol. 1971 Jul;107(1):106–113. doi: 10.1128/jb.107.1.106-113.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan J. E., Jr, Setlow R. B. Thymine Photoproducts but not Thymine Dimers Found in Ultraviolet-Irradiated Bacterial Spores. Science. 1965 Jul 16;149(3681):308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Postemsky C. J., Dignam S. S., Setlow P. Isolation and characterization of Bacillus megaterium mutants containing decreased levels of spore protease. J Bacteriol. 1978 Sep;135(3):841–850. doi: 10.1128/jb.135.3.841-850.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman Y., Fields M. L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1968 Jan;22(1):168–168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Quantum yield determinations of photosynthetic reactions. Methods Enzymol. 1972;24:139–146. doi: 10.1016/0076-6879(72)24064-2. [DOI] [PubMed] [Google Scholar]
- Setlow P. Deoxyribonucleic acid synthesis and deoxynucleotide metabolism during bacterial spore germination. J Bacteriol. 1973 Jun;114(3):1099–1107. doi: 10.1128/jb.114.3.1099-1107.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Identification and localization of the major proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8159–8167. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Primus G., Deutscher M. P. Absence of 3'-terminal residues from transfer ribonucleic acid of dormant spores of Bacillus megaterium. J Bacteriol. 1974 Jan;117(1):126–132. doi: 10.1128/jb.117.1.126-132.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Primus G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem. 1975 Jan 25;250(2):623–630. [PubMed] [Google Scholar]
- Setlow P. Purification and characterization of additional low-molecular-weight basic proteins degraded during germination of Bacillus megaterium spores. J Bacteriol. 1978 Oct;136(1):331–340. doi: 10.1128/jb.136.1.331-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Purification and properties of a specific proteolytic enzyme present in spores of Bacillus magaterium. J Biol Chem. 1976 Dec 25;251(24):7853–7862. [PubMed] [Google Scholar]
- Setlow P. Purification and properties of some unique low molecular weight basic proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8168–8173. [PubMed] [Google Scholar]
- Setlow P., Waites W. M. Identification of several unique, low-molecular-weight basic proteins in dormant spores of clastridium bifermentans and their degradation during spore germination. J Bacteriol. 1976 Aug;127(2):1015–1017. doi: 10.1128/jb.127.2.1015-1017.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Thermal denaturation and template properties of DNA complexes with purified histone fractions. J Mol Biol. 1970 Mar;48(3):469–487. doi: 10.1016/0022-2836(70)90059-8. [DOI] [PubMed] [Google Scholar]
- Stafford R. S., Donnellan J. E., Jr Photochemical evidence for conformation changes in DNA during germination of bacterial spores. Proc Natl Acad Sci U S A. 1968 Mar;59(3):822–828. doi: 10.1073/pnas.59.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]


