Abstract
The type II secretion (T2S) system of Vibrio cholerae is a multiprotein complex that spans the cell envelope and secretes proteins important for pathogenesis as well as survival in different environments. Here we report that, in addition to the loss of extracellular secretion, removal or inhibition of expression of the T2S genes, epsC-N, results in growth defects and a broad range of alterations in the outer membrane that interfere with its barrier function. Specifically, the sensitivity to membrane-perturbing agents such as bile salts and the antimicrobial peptide polymyxin B is increased, and periplasmic constituents leak out into the culture medium. As a consequence, the σE stress response is induced. Furthermore, due to the defects caused by inactivation of the T2S system, the Δeps deletion mutant of V. cholerae strain N16961 is incapable of surviving the passage through the infant mouse gastrointestinal tract. The growth defect and leaky outer membrane phenotypes are suppressed when the culture medium is supplemented with 5% glucose or sucrose, although the eps mutants remain sensitive to membrane-damaging agents. This suggests that the sugars do not restore the integrity of the outer membrane in the eps mutant strains per se but may provide osmoprotective functions.
Gram-negative bacteria possess highly sophisticated and organized cell envelopes that consist of inner and outer membranes separated by the periplasmic compartment and the peptidoglycan layer. The outer membrane is made up of a lipopolysaccharide (LPS)-phospholipid asymmetric bilayer and functions as a barrier preventing entry of toxic substances, including antibiotics, dyes, and detergents (60, 72). At the same time, the outer membrane allows nutrient acquisition and transport of molecules in and out of the cell. Several dedicated transport systems have evolved for this purpose, and at least six pathways are required for extracellular protein secretion alone (43, 67). One such system is the type II secretion (T2S) system, which has been identified in a wide variety of Proteobacteria, including many pathogens (for reviews, see references 12 and 74). Many of the proteins secreted by the T2S pathway, such as proteases, lipases, cellulases, pectinases, phospholipases, lipases, and toxins, contribute to virulence. These secreted proteins are synthesized with signal peptides and are first transported into the periplasmic compartment by Sec- or Tat-dependent processes and then cross the outer membrane through the T2S machinery (66, 89).
In recent years, much attention has been paid to the interactions between individual components and the mechanism by which the multiprotein T2S complex is assembled. The present model for T2S machines includes a secretion pore in the outer membrane, a multiprotein subcomplex localized in the cytoplasmic membrane, a pseudopilus that spans the periplasmic compartment, and an ATPase in the cytoplasm (38). Besides the interactions within the T2S complex, it appears that T2S components also interact with other cell envelope constituents. For example, it has been shown that an alteration in the LPS structure affects the function of the T2S system in Pseudomonas aeruginosa (6, 53). In addition, interaction with peptidoglycan is a prerequisite for the ExeAB complex to support assembly of the outer membrane protein ExeD in the Aeromonas hydrophila T2S system (34). It also has been demonstrated that T2S mutants of A. hydrophila, V. cholerae, and Vibrio sp. strain 60 possess altered outer membrane protein profiles in addition to defects in extracellular secretion (33, 35, 37, 77). The amounts of outer membrane proteins, including OmpU, OmpT, and the LamB homolog OmpS, are considerably diminished in eps mutants of V. cholerae (64, 77). Similarly, the levels of OmpS and OmpF porins are reduced in exe mutants of A. hydrophila (33). The reason for these outer membrane defects is not known but might suggest a role for the T2S machinery in outer membrane biogenesis (33, 37, 77). The outer membrane alterations might be responsible for the slow-growth phenotype noted for T2S mutants of V. cholerae (39, 64, 77). Although no outer membrane changes have been reported for T2S mutants of Legionella pneumophila, they also display reduced growth rates under certain growth conditions (82).
In this study, we have taken a closer look at the effect of inactivation of the T2S machinery on outer membrane integrity in V. cholerae. We show that removal or inactivation of the Eps system results in a compromised outer membrane evidenced by several phenotypes, including extracellular release of periplasmic content and sensitivity to bile salts and polymyxin B. We also show that V. cholerae lacking a functional Eps system is unable to survive the passage through the infant mouse intestine and is rapidly cleared.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains used in this study are listed in Table 1. Strains were cultured in Luria-Bertani (LB) broth at 37°C, supplemented as specified in the text.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotypea | Reference or source |
|---|---|---|
| Strains | ||
| V. cholerae | ||
| O395 | Classical O1 biotype, Smr | Laboratory collection |
| Mut3 | O395 epsE::lacZ kan Smr Kanr | 77 |
| JJM43 | O395 ΔtoxR43 ΔctxA1 Smr | 27 |
| Mut4 | O395 ΔtoxR43 ΔctxA1 epsE::lacZ kan Smr | 77 |
| O395ΔrpoE | O395ΔrpoE Smr | 49 |
| TRH7000 | El Tor O1 biotype, thy Hgr Δ(ctxA-ctxB) | 29 |
| Mut1 | TRH7000 epsE::lacZ kan Kanr | 75 |
| Mut6 | TRH7000 epsF::kan Kanr | 75 |
| Mut5 | TRH7000 epsG::kan Kanr | 77 |
| Mut 8 | TRH7000 epsL::kan Kanr | 75 |
| PU3 | TRH7000 epsM::Tn5 Kanr | 62 |
| TRHΔepsC | TRH7000 ΔepsC Kanr | S. R. Lybarger et al. (unpublished data) |
| TRHΔepsL | TRH7000 ΔepsL Kanr | This study |
| PBAD::eps | TRH7000 PBAD::eps | This study |
| TRHΔeps | TRH7000 ΔepsC-N Cmr | This study |
| N16961 | Wild-type El Tor O1 biotype, Smr | Laboratory collection |
| NΔeps | N16961 ΔepsC-N Cmr | This study |
| 569B | Classical O1 biotype Smr | Laboratory collection |
| M14 | 569B epsE-A321V | 32 |
| Bah2 | El Tor O1 biotype, E7946, ΔattRS | 87 |
| Bah2 epsD::kan | Bah2 epsD::kan | 14, 15 |
| E. coli | ||
| MC1061 | F−araD139 Δ(ara-leu)7697 Δ(lac)X74 rpsL hsdR2 mcrA mcrB1 | 11 |
| S17.1 | F− recA pro hsdR RP4-2 Tcr::Mu Tnr::Tn7 | 81 |
| SY327 λpir | Δ(lac-pro) argE(Am) recA56 rpoB λpir Rifr | 55 |
| MM294 (pRK2013) | Donor of transfer function for triparental conjugation | 52 |
| Plasmids | ||
| pPCR-Script | Cloning vector, Ampr | Stratagene |
| pBAD33 | Cloning vector, araC-PBAD Cmr | 25 |
| pJN105 | Cloning vector, ori pBBR1 araC-PBAD mob Gmr | 59 |
| pK18mobsacB | ori-pMB1 oriT (RP4) sacB lacZα Kanr | 79 |
| pMMB-GFP | pMMB66-gfp coding sequence, Ampr | 80 |
| pMMB68 | etxB under PTAC control in pMMB66EH, Ampr | 76 |
| pMMB190 | Cloning vector, pTACplacUV5 Ampr | 57 |
| pCH40 | pbla::phoA Tetr | 31 |
| pMS43 | epsE-K270A under PTAC control in pMMB207, Cmr | 14, 75 |
| pCVD442 | ori R6K mobRP4 sacB Ampr | 17 |
| pUC18K | Cloning vector, aphA-3 Ampr Kanr | 51 |
| pBBRlux | ori pBBR1, promoterless luxCDABE Cmr | 46 |
| pAES100 | 1,078-bp EcoRI-XbaI fragment upstream of epsC in pK18mobsacB, Kanr | This study |
| pAES101 | 1,075-bp fragment downstream of epsN cloned into XbaI- and SphI-digested pAES100, Kanr | This study |
| pAES102 | 923-bp fragment containing Cmr cassette cloned into XbaI site of pAES101, Kanr Cmr | This study |
| pAES103 | 1,070-bp fragment upstream of epsC cloned into SphI-ClaI site of pJN105, Gmr | This study |
| pAES104 | 1,079-bp fragment of full-length, promoterless epsC cloned into EcoRI- and XbaI-cut pAES103, Gmr | This study |
| pAES105 | SphI-XbaI 3,479-bp fragment containing upstream region of epsC, araC, and pBAD::epsC from pAES104 in pK18mobsacB, Kanr | This study |
| pAES106 | rpoEP2 cloned into pBBRlux, Cmr | This study |
| pMMB131 | pMMB68 gene fusion between etxB signal sequence and phoA under PTAC control, Ampr | This study |
| pEps | 15-kb XbaI-SphI fragment containing entire eps operon (from epsC to epsN) in pMMB190, Ampr | This study |
Ampr, ampicillin resistance; Cmr, chloramphenicol resistance; Gmr, gentamicin resistance; Hgr, mercury resistance; Kanr, kanamycin resistance; Rifr, rifampin resistance; Tetr, tetracycline resistance.
V. cholerae TRH7000 and its mutant derivatives were cultured in the presence of thymine (100 μg/ml) (29). Lipid agar was used to determine the export of lipase on solid agar. The medium was prepared as described previously (40) with modified minimal medium (47.75 mM Na2HPO4, 22 mM KH2PO4, 8.5 mM NaCl, 18.69 mM NH4Cl, 0.1% Tween 20, 0.2 mM CaCl2) containing olive oil as the only source of carbon.
Antibiotics (Sigma-Aldrich, St. Louis, MO) were used at the following concentrations: chloramphenicol, 4 or 10 μg/ml for chromosomal or plasmid expression, respectively, for V. cholerae and 30 μg/ml for Escherichia coli; carbenicillin, 100 μg/ml; kanamycin, 50 μg/ml; streptomycin, 100 μg/ml; and polymyxin B sulfate, 100 U/ml.
Construction of Δeps, PBAD::eps, and ΔepsL strains.
Chromosomal DNA isolated from V. cholerae TRH7000 or N16961 was used as the template for PCR where indicated. PCRs were carried out with PfuUltra DNA polymerase (Stratagene, La Jolla, CA). Primers were synthesized by IDT Technologies, Inc. (Coralville, IA). All PCR products were first subcloned into pPCR-Script Amp SK(+) (Stratagene) and then processed further.
The Δeps strains were generated by PCR amplification of the upstream region of the epsC gene with primers 5′-GAATTCTTGCTCGAAATCTTCAGCTG-3′ (forward [Fwd]) and 5′-TCTAGACGTTAAATTGTCGACTTGC-3′ (reverse [Rev]). The resulting 1,078-bp fragment was digested with EcoRI and XbaI and ligated into pK18mobsacB (79) that had been likewise digested to yield pAES100. The region downstream of the epsN gene (the last gene in the eps operon) was amplified using primers 5′-TCTAGAATCCCATTCATTTCACGC-3′ (Fwd) and 5′-GCATGCTGTTGCTCAGGATCTATAC-3′ (Rev). The resulting fragment of 1,075 bp subsequently was digested with XbaI and SphI and ligated into similarly cut pAES100 to yield pAES101. The chloramphenicol cassette was obtained by amplification of the cat gene from pBAD33 (25) with primers 5′-TCTAGATAGCACCAGGCGTTTAAG-3′ (Fwd) and 5′-TCTAGAGCGCCGAATAAATACCTG-3′ (Rev), and the resulting product was digested with XbaI. The 923-bp fragment was ligated into XbaI-digested pAES101 to form pAES102, which then was conjugated from the E. coli strain S17.1 into the V. cholerae N16961 and TRH7000 strains as described previously (81, 88). Transconjugants in which pAES102 had recombined into the respective V. cholerae genomes were selected on LB agar containing chloramphenicol. To select for the second recombination event, independent colonies of TRH7000 and N16961 cointegrants were cultured overnight in LB broth supplemented with chloramphenicol, diluted, and cultured to late log phase and spread on LB agar containing 10% sucrose and no NaCl. Chloramphenicol-resistant colonies were screened on LB agar containing 1% milk for the loss of plasmid-encoded kanamycin resistance and for the loss of protease secretion. Colonies that were kanamycin sensitive, chloramphenicol resistant, and protease negative were designated TRHΔeps and NΔeps for V. cholerae strains TRH7000 and N16961, respectively.
The PBAD::eps strain was constructed as follows. The upstream region of the epsC gene was amplified using primers 5′-GCATGCTGCTCGAAATCTTCAG-3′ (Fwd) and 5′-ATCGATACGTTAAATTGTCGACTTGC-3′ (Rev) and was digested with SphI and ClaI. The 1,070-bp fragment was cloned into similarly digested pJN105 (59) to yield pAES103. Chromosomal DNA containing promoterless epsC was amplified using primers 5′-GCGAATTCAGTACAGAAAGGAATAACG-3′ (Fwd) and 5′-TCTAGACTAGAGGGGTAGACAGC-3′ (Rev) and was digested with EcoRI and XbaI. The 1,079-bp fragment was cloned into EcoRI/XbaI-cut pAES103 to yield pAES104, resulting in the placement of the epsC gene behind the PBAD promoter. The 3,479-bp SphI-XbaI fragment containing the upstream region of the epsC gene, araC, and PBAD::epsC was moved from pAES104 and ligated into pK18mobsacB (79). The resulting plasmid, pAES105, subsequently conjugated from the E. coli strain S17.1 (81, 88), was employed to introduce the mutation onto the V. cholerae THR7000 chromosome. Conjugants were plated on LB agar supplemented with kanamycin and arabinose (0.01%).
To select for the second recombination event, everything was completed as described above with the following modifications. Independent colonies of transconjugants were cultured in LB broth supplemented with arabinose (0.01%) to late log phase and were spread on LB agar containing 10% sucrose, 0.01% arabinose, and no NaCl. All colonies were screened for the loss of kanamycin resistance after being patched on LB agar containing 1% milk with or without 0.01% arabinose. Clones that did secrete protease in the presence, but not in the absence, of arabinose were selected for further analysis.
To generate the TRHΔepsL strain, 1-kb regions upstream and downstream of the epsL gene were amplified using primer pairs 5′-GAGCTCTTAATTGTGATTCTGCTCCT-3′ (Fwd) and 5′-GGTACCAAACCAGCCAAGGGATATC-3′ (Rev) as well as 5′-TCTAGAGTTTGTGGTGAAGCCCAAG-3′ (Fwd) and 5′-GTCGACAAGCTAAGCTGCCTTCG-3′ (Rev) and were cloned into pUC18K (51). This resulted in a sequence containing aph-3, which confers kanamycin resistance, flanked by homologous regions for recombination and allelic exchange. The entire fragment was excised using flanking restriction enzyme sites and was cloned into pCVD442 (17). The suicide vector was propagated in SY327 λpir (55) and was conjugated into TRH7000 with the assistance of MM294/pRK2013 (52). Carbenicillin-resistant transconjugants resulting from the integration of the suicide vector onto the chromosome were isolated. Positive integrants then were grown nonselectively in LB broth and were plated on LB agar without NaCl (supplemented with 10% sucrose) to facilitate the second recombination event. Colonies from the sucrose agar were scored on LB agar containing either kanamycin or carbenicillin and 1% nonfat milk to visualize protease secretion.
Following the selection procedures described above, deletion of the eps operon and the epsL gene as well as the exchange of the region containing the eps promoter were confirmed by PCR, sequencing (at the University of Michigan DNA Sequencing Core), and Western blotting with antisera against Eps proteins.
Growth curve experiments.
Equivalent dilutions (based on the optical density at 600 nm [OD600]) of overnight LB broth cultures of wild-type and eps mutant V. cholerae strains grown with or without different saccharides, including glucose, sucrose, maltose, mannose, lactose, galactose, sorbitol, melibiose, raffinose, xylose, and arabinose (up to 10% final concentration), or osmoprotectants, such as glycine-betaine and proline (up to 500 mM final concentration), or NaCl (up to 350 mM) were used as the inocula for cultures. The growth at 37°C was monitored over time by OD600 measurements at 30-min intervals. All experiments were performed in triplicate.
Antimicrobial assays.
To determine the lowest concentration of polymyxin B sulfate or bile salts inhibiting CFU to 50% (MIC50), overnight cultures of wild-type and eps mutants of V. cholerae were diluted 1:100 in LB broth and grown to mid-log phase (OD600 of approximately 0.5). To quantify the number of CFU, 10-fold dilutions of cultures were plated using Spiral Tech (Spiral Biotech, Norwood, MA) on a series of LB agar plates containing increasing concentrations of polymyxin B sulfate (0, 25, 50, 100, 200, 400, 600, 800, and 1,000 U/ml; Sigma-Aldrich) or bile salts (0, 0.0001, 0.0005, 0.001, 0.005, 0.01, 0.05, 0.1, 0.2, and 0.4%; Sigma-Aldrich). Colonies were counted using Q-Quant (Spiral Biotech) after growth at 37°C for 18 h. Mutant colonies were counted following 48 h of incubation due to slower growth. All assays were performed at least in triplicate.
Enzymatic assays.
For enzymatic assays, wild-type and mutant strains were grown in LB broth supplemented with isopropyl-β-d-thiogalactopyranoside (IPTG) (at a 50 μM final concentration) to induce the expression of plasmid-encoded green fluorescent protein (GFP), β-lactamase, or alkaline phosphatase (AP). Following growth for 16 h at 37°C (late-stationary-phase cultures; OD600 of approximately 4), culture media were separated from the cells by centrifugation (10 min, 8,000 rpm) and passed through 0.22-μm filters. Periplasmic extracts were obtained by incubating cells in 50 mM Tris, pH 8.0, with 2,000 U/ml of polymyxin B sulfate for 30 min (63). The spheroplasts were pelleted, and the supernatant was saved as a periplasmic extract.
A spectrophotometric assay was used to measure AP activity (22, 70) by incubating culture supernatants and periplasmic extracts with p-nitrophenylphosphate (Sigma-Aldrich) at 0.1 mg/ml in 50 mM Tris, pH 8.0, 1 mM MgCl2 for 15 min at 37°C. The assays were stopped by adding EDTA to a final concentration of 0.05 M, and the absorbance at 405 nm was measured for each sample.
β-Lactamase activity was determined by incubating culture supernatants and periplasmic extracts with nitrocefin (EMD Chemicals, San Diego, CA) at 0.1 mg/ml in 50 mM Tris, pH 8.0, 1 mM MgCl2 for 5 min at 37°C and measuring the absorbance at 482 nm (28).
GFP fluorescence in culture supernatants and whole-cell extracts was measured by excitation and emission at 485 and 535 nm, respectively (18).
All enzymatic assays were performed in quadruplicate. The percentage of the total amount detected in the supernatants was calculated, and means and corresponding standard errors of the means (SEM) are presented.
To determine the level of protease secretion, filtered supernatants from overnight cultures (16 h; OD600 of approximately 4) were assayed in 5 mM HEPES, pH 7.5, and 0.05 mM N-tert-butoxy-carbonyl-Gln-Ala-Arg-7-amido-4-methyl-coumarin (Sigma-Aldrich) for 10 min at 37°C using the excitation and emission wave lengths of 385 and 440 nm, respectively (10). The assay was performed in triplicate, and means and SEM are presented.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), silver staining, and Western blotting.
Samples of whole-cell lysates, culture supernatants, or periplasmic extracts prepared as described above and matched by equivalent OD600s were boiled in SDS sample buffer and analyzed on 4 to 12% Bis-Tris polyacrylamide gels (NuPAGE; Invitrogen). The gels subsequently were silver stained using a SilverQuest silver staining kit (Invitrogen) according to the manufacturer's instructions or were transferred to nitrocellulose (BioTraceNT; Pall Corporation, Pensacola, FL) in NuPAGE transfer buffer (Invitrogen). The blots were incubated with polyclonal antisera against V. cholerae OmpU (1:30,000; kind gift of K. Klose) or monoclonal anti-EtxB (1:30,000 [76]). Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:10,000; Bio-Rad, Hercules, CA) and rabbit anti-mouse immunoglobulin G (1:3,000; Bio-Rad) were used as the secondary antisera, and the blots were developed with the SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL) or ECL plus Western blotting detection reagent (GE Healthcare, Buckinhamshire, United Kingdom).
Construction of rpoEP2-lux reporter fusion and monitoring of bioluminescence.
A transcriptional rpoEP2-lux reporter was constructed based on an earlier study (44). Briefly, the rpoEP2 promoter region of V. cholerae N16961 chromosomal DNA was amplified using primers 5′-GGATCCAGATATGGGTACTCATTATG-3′ (Fwd) and 5′-ACTAGTCTGCCTTGATAAGGTTTTGAGTAAA-3′ (Rev). The amplified 142-bp fragment was cloned into BamHI-SpeI-digested pBBRlux containing promoterless luxCDABE, kindly supplied by Brian Hammer and Bonnie Bassler (46), to yield pAES106. The rpoEP2::lux fusion was verified by sequencing. Wild-type, PBAD::eps, ΔepsL, and ΔepsL+pEpsL strains of V. cholerae TRH7000 containing pAES106 were cultured in LB broth, supplemented with arabinose where indicated, to mid-log phase (OD600 of approximately 0.5) or stationary phase (OD600 of approximately 4). Serial dilutions of the cell cultures were transferred to 96-well white opaque microtiter plates (Lumitrac), and bioluminescence was measured using a multidetection microplate reader (SynergyHT; Bio-Tek Instruments, Winoosh, VT). Bioluminescence was standardized for cell density by dividing the arbitrary light units by the absorbance at 600 nm. The data represent average values and corresponding SEM of three independent experiments (in which each experiment was performed in three technical replicates).
Infant mouse studies.
Overnight cultures of wild-type and NΔeps strains of V. cholerae N16961 were administered into anesthetized 4- to 5-day-old suckling CD-1 mice (3) in a peroral inoculum of 106 and 107 to 108 cells, respectively, in accordance with University of Michigan Committee on Use and Care of Animals guidelines. Mice were separated from their mothers and were kept at 30°C for 24 h, at which time they were euthanized, their intestines were isolated and homogenized in 5 ml of LB broth, and the number of CFU was determined by plating dilutions on LB agar with streptomycin.
Statistical analysis.
A Student's t test was applied for all statistical analyses, and values were considered significant at P < 0.05.
RESULTS
Alterations in the Eps system cause a leaky outer membrane.
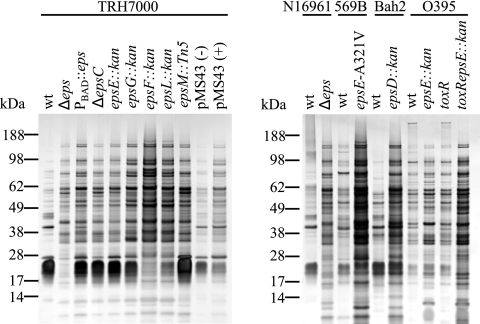
Inactivation of the V. cholerae T2S system results in growth defects (39, 64, 77) and an aberrant outer membrane protein profile (64, 77). In order to address the consequences of the outer membrane alterations in V. cholerae and to test the hypothesis that the integrity of the outer membrane is compromised by T2S disruption, we analyzed the protein content of the culture supernatant isolated from late-stationary-phase cultures of wild-type and eps mutant strains grown in LB broth at 37°C by SDS-PAGE and silver staining (Fig. 1). Wild-type and mutant strains displayed very different extracellular protein profiles, and the numbers of protein species as well as their amounts were dramatically increased in the culture supernatant of the eps mutants. This unusual phenomenon, generally not expected of secretion mutants, was observed regardless of biotype (classical or El Tor), ToxR (virulence gene regulator) status, or specific eps mutant genotype. Similar observations also were made when a plasmid-encoded dominant-negative mutant of EpsE, EpsE-K270A, was expressed in the wild-type strain TRH7000. In addition to blocking toxin secretion (14, 75), overproduction of the mutant EpsE-K270A protein following addition of IPTG resulted in increased leakage of proteins to the culture supernatant of the wild-type strain as shown in Fig. 1.
FIG. 1.
Increased protein content in the culture supernatant of V. cholerae eps mutants. Wild-type (Wt) and mutant V. cholerae strains (with mutated alleles indicated in italics) were grown in LB broth at 37°C to stationary phase (16 h). Culture supernatants were separated from cells by centrifugation, matched by equivalent OD600 units, and analyzed by SDS-PAGE and silver staining. The El Tor biotype is represented by V. cholerae strains TRH7000, N16961, and Bah2. Strains 569B and 0395 are representatives of the classical biotype. Plasmid pMS43-encoded EpsE-K270A (a dominant-negative mutant protein) was expressed in wild-type TRH7000 by growth in the absence (−) or presence (+) of 10 μM IPTG. The migration of molecular mass markers is indicated.
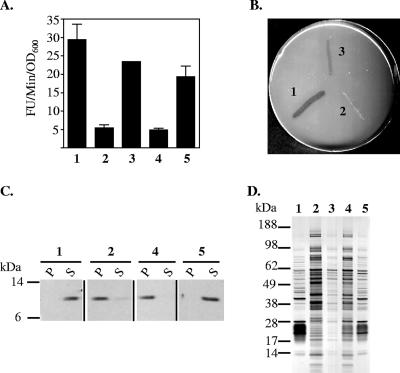
In order to determine whether the outer membrane defects were due to the loss of T2S and not simply due to uncontrolled activities of the remaining Eps proteins, additional mutants were constructed. The mutations were introduced into TRH7000, a nontoxigenic V. cholerae strain in which most eps mutations have been constructed (77), and N16961, a virulent strain that can be administered into infant mice for colonization studies (3, 21). The chromosomal region encoding the 12 Eps proteins was replaced with a chloramphenicol cassette by homologous recombination in V. cholerae strains TRH7000 and N16961, resulting in strains designated TRHΔeps and NΔeps, respectively. Additionally, we generated a strain of V. cholerae TRH7000, designated PBAD::eps, in which the chromosomal region containing the native eps promoter was replaced with the arabinose-inducible promoter (PBAD). As expected, complete removal of the eps genes or growth of the PBAD::eps mutant in the absence of arabinose prevented extracellular secretion of several proteins (Fig. 2). There was a sixfold reduction in the level of extracellular protease of the mutants, as determined by the rate of N-tert-butoxy-carbonyl-Gln-Ala-Arg-7-amido-4-methyl-coumarin hydrolysis (Fig. 2A). The ability to secrete lipase was tested on lipid agar medium (40). Wild-type V. cholerae grows on this medium by secreting a lipase that is capable of hydrolyzing an emulsion of olive oil and uses the hydrolyzed products as a sole carbon and energy source. In contrast, TRHΔeps was not capable of growth on this medium, likely due to its inability to secrete the lipase (Fig. 2B). Finally, to determine the level of toxin secretion in the mutant strains, culture supernatants and periplasmic extracts isolated from wild-type and mutant TRH7000 cells that express EtxB, the B subunit of the E. coli heat-labile enterotoxin (which is secreted with the same mechanism as that for cholera toxin [30]), were analyzed by SDS-PAGE and immunoblotting with monoclonal anti-EtxB (Fig. 2C). While EtxB was present in the supernatants of the wild-type strain and the PBAD::eps mutant grown in the presence of 0.01% arabinose, EtxB accumulated in the periplasm of the TRHΔeps mutant and the PBAD::eps strain grown in the absence of arabinose. The secretion of protease, lipase, and EtxB was restored in the TRHΔeps mutant in the presence of a plasmid, pEps, that carries the entire eps operon or by adding arabinose to a final concentration of 0.01% to the growth medium of the PBAD::eps strain (Fig. 2A to C).
FIG. 2.
Inverse correlation between T2S and passive leakage. Wild-type and eps mutant strains of V. cholerae TRH7000 were analyzed for the secretion of protease, lipase, and toxin, as well as for the release of cellular proteins to the extracellular environment. (A) Culture supernatants were obtained from stationary-phase cultures as described in Materials and Methods and were tested for the presence of extracellular protease using the proteolytic substrate N-tert-butoxy-carbonyl-Gln-Ala-Arg-7-amido-4-methyl-coumarin. The rate of hydrolysis (relative fluorescence units [FU]/min/OD600 unit) is presented as the means of three independent experiments ± SEM. There was a statistically significant difference between the protease activity of wild-type and eps mutant strains (P < 0.005). (B) Lipase secretion was determined by growth on lipid agar containing olive oil as the only source of carbon. (C) The distribution of the E. coli heat-labile enterotoxin B subunit in culture supernatants (S) and periplasmic extracts (P) was determined by SDS-PAGE and immunoblotting using monoclonal anti-EtxB. (D) Culture supernatants were obtained from stationary-phase cultures, matched by equivalent OD600 units, and analyzed by SDS-PAGE and silver staining for total protein content. Lane 1, wild type; lane 2, TRHΔeps; lane 3, TRHΔeps+pEps; lane 4, PBAD::eps; lane 5, PBAD::eps grown in the presence of arabinose at a 0.01% final concentration.
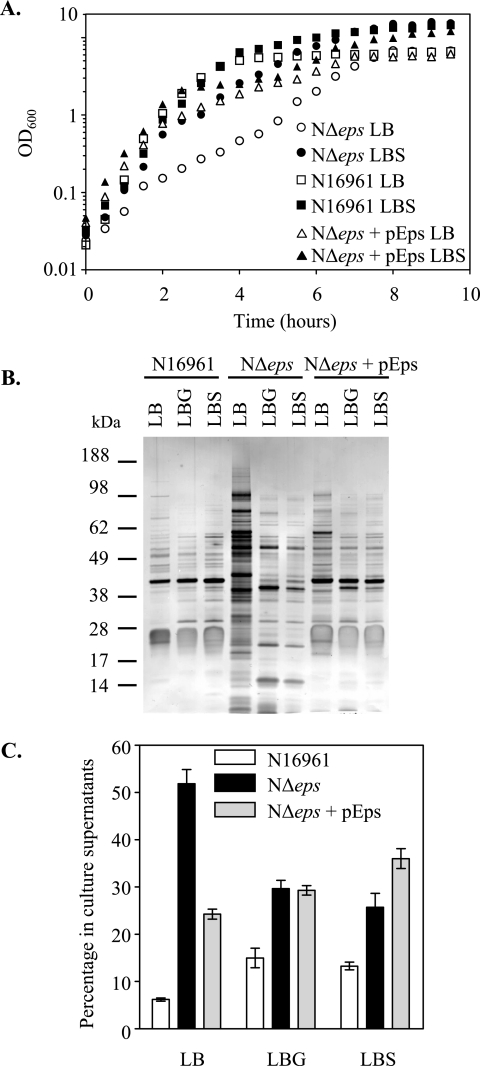
Similar to the single-gene-deletion mutants, a small-colony phenotype also was observed for the Δeps mutants and the PBAD::eps strain grown without arabinose regardless of growth temperature (20, 30, and 37°C), and the doubling time in LB broth at 37°C increased from 20 min for the wild-type strain N16961 to 90 min for the NΔeps mutant (see Fig. 6A). The ability to retain intracellular proteins also was compromised in both the TRHΔeps and NΔeps strains, as well as the PBAD::eps strain grown in the absence of arabinose, as the protein profile of the growth medium from these mutants contained an increased level of proteins (Fig. 1 and 2D). This suggests that the permeability of the outer membrane is increased when the Eps system is inactivated, while the active secretion of Eps-dependent proteins, including protease, lipase, and toxin, is diminished (Fig. 2). As expected, the leaky outer membrane phenotype could be corrected with the pEps plasmid that encodes all the eps genes or by the addition of arabinose to the culture of the PBAD::eps strain (Fig. 2D).
FIG. 6.
Sucrose and glucose suppress the growth defect and leaky phenotype of the Δeps mutant strain. (A) Growth of wild-type, NΔeps, and NΔeps+pEps strains of V. cholerae N16961 in LB broth or LB broth supplemented with sucrose (LBS) at a 5% final concentration was monitored at OD600. (B) Culture supernatants were isolated from wild-type, NΔeps, and NΔeps+pEps strains grown in LB broth, LBG (LB with glucose at a 5% final concentration), or LBS and analyzed by SDS-PAGE and silver staining. (C) Culture supernatants and periplasmic fractions of wild-type, NΔeps, and NΔeps+pEps cells grown in LB broth, LBG, or LBS were assayed for the presence of β-lactamase as described in Materials and Methods. The level of β-lactamase in the culture supernatant was expressed as a percentage of the total amount of the enzyme present in both the culture supernatant and cellular extract. The means of at least three independent experiments and corresponding SEM are presented. There was a statistically significant decrease in leakage of β-lactamase to the culture supernatant of NΔeps grown in LBG or LBS medium compared to the leakage of NΔeps grown in LB medium (P < 0.0005).
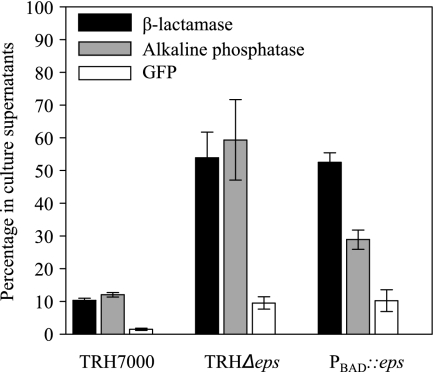
The outer membrane integrity of the TRHΔeps and PBAD::eps strains was further assessed by specifically determining the distribution of periplasmic and cytoplasmic protein markers present in the culture supernatant following growth for 16 h (to an approximate OD600 of 4). The majority (90%) of plasmid-encoded β-lactamase and AP produced by the wild-type TRH7000 strain remained periplasmic, while 55 to 65% of the total content of these periplasmic enzymes escaped to the culture media from the TRHΔeps and PBAD::eps mutants cells (Fig. 3). In addition, there were detectable levels (12% of the total amount) of the cytoplasmic GFP in the culture supernatant of the mutants, suggesting that there is also an increase in cell lysis in the late stationary phase of growth (Fig. 3).
FIG. 3.
Inactivation of the Eps pathway results in the leakage of periplasmic proteins. Culture supernatants and periplasmic as well as cytoplasmic extracts from cells present in overnight cultures of wild-type TRH7000, TRHΔeps, and PBAD::eps (grown in the absence of arabinose) were assayed for the presence of β-lactamase, AP, or GFP as described in Materials and Methods. The level of each of these proteins in the culture supernatant was expressed as a percentage of the total amount of each protein present in both culture supernatant and cellular extract. The means of four independent experiments ± SEM are presented. For all proteins tested, P < 0.02.
Although eps mutants release a greater number of protein species than the wild-type strains, several proteins present in the wild-type culture medium are absent in the supernatant of the eps mutants (Fig. 1 and 2D). Some of them likely represent cholera toxin, lipase, and protease, but the 38-, 26-, and 22-kDa species may correspond to yet-to-be identified T2-secreted proteins. To identify these proteins, culture supernatants were acetone precipitated, followed by SDS-PAGE and Coomassie staining (data not shown). The three bands representing proteins with molecular masses corresponding to 38, 26, and 22 kDa present in wild-type but not NΔeps supernatants were excised and subjected to tandem mass spectrometry (at the Michigan Proteome Consortium). These proteins turned out to be outer membrane proteins, not extracellularly secreted proteins, and were identified as OmpU, OmpV (85), and OmpW (36, 58). Their presence in the wild-type culture supernatant likely is due to the release of outer membrane fragments and/or vesicles during growth overnight, as immunoblotting revealed that OmpU could be removed from the culture supernatant by centrifugation at 170,000 × g (data not shown). The reduced level of OmpU in the culture supernatant of the NΔeps mutant likely reflects its overall reduced intracellular level (see Fig. 7). The lack of OmpV and OmpW in the NΔeps culture supernatant also may be due to their reduced levels in the outer membrane of the NΔeps mutant.
FIG. 7.
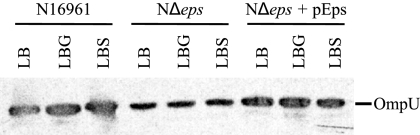
Reduced level of OmpU in Δeps mutants. Western blot of cellular extracts obtained from cultures of wild-type, NΔeps, and NΔeps+pEps strains of V. cholerae N16961 grown in LB broth or in LB broth supplemented with glucose (LBG) or sucrose (LBS) to a 5% final concentration for 16 h at 37°C. Samples were matched by equivalent OD600 units, separated by SDS-PAGE, transferred to nitrocellulose, and probed with OmpU antiserum.
Inactivation of the Eps system results in increased sensitivity to antimicrobial agents.
The aberrant outer membrane protein profile and the increased outer membrane permeability of the eps mutants suggested that they are hypersensitive to membrane-perturbing agents such as antimicrobial peptides and detergents. Polymyxin B, a peptide antibiotic, binds to lipid A and is lethal to many gram-negative bacteria (for a review, see reference 26). To test the hypothesis that the eps mutants exhibit an increased susceptibility to polymyxin B, we determined the MIC50 of polymyxin B for wild-type and mutant cells. Specifically, mid-log-phase cultures (at an OD600 of ∼0.5) of wild-type N16961 and the isogenic NΔeps strain were spread on LB agar supplemented with increasing concentrations of polymyxin B, as described in Materials and Methods. Polymyxin B, at 800 U/ml, reduced the CFU of the wild-type strain by 50%. For comparison, the MIC50 for the NΔeps mutant was as low as 100 U/ml (Table 2). Although OmpU has been implicated in polymyxin B resistance in V. cholerae (48), ΔompU strains are capable of selective growth in the presence of this cationic peptide (64). Likewise, V. cholerae strain TRH7000 lacks detectable levels of OmpU, yet it is capable of growth at several hundred units of polymyxin B per milliliter (77), and the MIC50 for polymyxin B was only reduced to 400 U/ml (Table 2). Compared to the wild-type cells of TRH7000, both the TRHΔeps and the PBAD::eps strains exhibited a fourfold increase in sensitivity to this cationic peptide (Table 2). Resistance to polymyxin B was restored to wild-type levels in the TRHΔeps and NΔeps mutants with the plasmid carrying the entire eps operon or in the PBAD::eps mutant grown in the presence of arabinose (Table 2). Finally, the same level of sensitivity to polymyxin B was observed with single-gene-deletion mutant TRHΔepsL (Table 2), indicating that different types of eps mutants display similar phenotypes. The sensitivity to polymyxin B was reversed in TRHΔepsL by expressing plasmid-encoded EpsL.
TABLE 2.
Effect of eps mutation on antimicrobial resistance
| Strain | MIC50a
|
|
|---|---|---|
| Polymyxin B (U/ml) | Bile salts (%) | |
| N16961 | ||
| Wild type | 800 | 0.05 |
| NΔepsc | 100 | 0.0005 |
| NΔeps+pEps | 800 | 0.05 |
| TRH7000 | ||
| Wild type | 400 | NDb |
| TRHΔepsc | 100 | ND |
| PBAD::epsc | 100 | ND |
| TRHΔeps+pEps | 400 | ND |
| PBAD::eps+arabinose | 400 | ND |
| TRHΔepsLc | 100 | ND |
| TRHΔepsL+pEpsL | 400 | ND |
The assays were conducted at least three times, and all standard deviations were less than 15%.
ND, not determined.
Colonies were counted after 48 h of incubation at 37°C.
Bile normally is present in the small intestine, and resistance to this detergent-like agent is important for the survival of enteric pathogens. The global virulence regulator ToxR mediates enhanced bile resistance in V. cholerae (65). As TRH7000 produces no detectable ToxR (77), we tested the wild-type strain and NΔeps mutant of N16961 to determine the lowest concentration of bile salts (sodium cholate/deoxycholate at a 1:1 ratio) that inhibits CFU to 50%. The mutant strain displayed a severe growth defect in the presence of bile salts, and the MIC50 was reduced 100-fold. The resistance to bile salts was restored when the mutant strain was complemented (Table 2).
NΔeps mutant does not survive the mouse gastrointestinal tract.
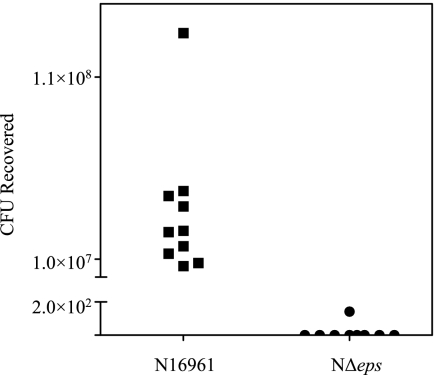
Because the NΔeps mutant was sensitive to an antimicrobial peptide and bile salts, we speculated that it may have difficulties surviving the intestinal environment of the infant mouse, a model for V. cholerae infection (3, 21). To test this hypothesis, overnight cultures of the NΔeps mutant and its isogenic wild-type V. cholerae strain, N16961, were separately inoculated perorally into 5-day-old mice. Twenty-four hours later, the intestines were excised and homogenized and were plated on LB agar to determine the number of CFU (Fig. 4). The number of CFU for the wild-type strain increased sevenfold following intestinal colonization (from 8.4 × 106 in the inoculum to an average of 5.8 × 107 in five mice in the first experiment and from 1.8 × 106 in the inoculum to an average of 1.16 × 107 in a total of five mice in the second experiment). The opposite trend was observed following inoculation of the NΔeps mutant. In the first experiment, out of four inoculated mice, NΔeps cells were recovered from one mouse only at 143 CFU, whereas 3.2 × 107 CFU were recovered from the inoculum. In the second experiment the input for the mutant strain was increased to 2.2 × 108, but no culturable bacteria were recovered from any of the five infected animals. The combined results from the two independent experiments conducted for both the wild-type and mutant strains presented in Fig. 4 strongly suggest that the intestinal environment is not conducive to mutant growth, and therefore the NΔeps mutant cells were cleared from the gastrointestinal tract of the inoculated mice.
FIG. 4.
NΔeps mutant does not survive the passage through the infant mouse gastrointestinal tract. Wild-type and NΔeps mutant strains of V. cholerae N16961 were separately inoculated into 4- to 5-day-old suckling mice as described in Materials and Methods. After 24 h postinoculation, the small intestines of the infected animals were removed and homogenized in LB broth, and serial dilutions were plated on LB agar supplemented with streptomycin to determine the number of CFU. The combined results of two independent experiments are presented. Datum points represent the CFU of wild-type N16961 (squares) and NΔeps mutant (circles) recovered from individual mice.
Induction of rpoE promoter activity in T2S mutants.
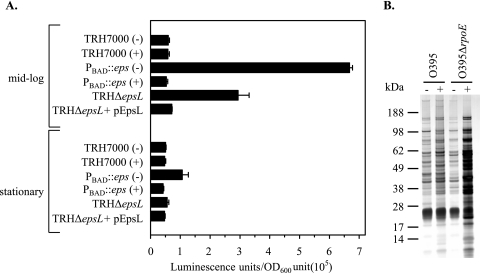
Homeostasis of the bacterial cell envelope is governed by several extracytoplasmic response systems, which are activated by distinct signals (19, 73). The alternative sigma factor, σE (RpoE), is activated in response to misfolded outer membrane proteins and envelope damage and controls the expression of several periplasmic chaperones, proteases, LPS biosynthesis proteins, and outer membrane proteins that ensure proper outer membrane assembly (2, 72). As activation of the σE stress response is an indication of outer membrane damage, we reasoned that increased σE activity in the eps mutants would be another means by which damage to the outer membrane can be demonstrated. Therefore, a luciferase reporter gene fusion was constructed to determine whether eps mutants display enhanced σE activity. As in E. coli (68, 69, 71), the rpoE gene of V. cholerae is under the control of two promoters, a distal σ70-dependent P1 and P2, located immediately upstream of rpoE. The P2 promoter matches the σE consensus sequence and is positively regulated by RpoE (44). The P2 promoter region was cloned upstream of the luciferase gene to monitor the promoter activity, and the resulting rpoEP2::lux transcriptional fusion plasmid was introduced into wild-type, PBAD::eps, ΔepsL, and ΔepsL+pEpsL strains of V. cholerae TRH7000. When mid-log-phase cultures (OD600 ∼ 0.5) were examined, PBAD::eps grown in the absence of arabinose exhibited 11-fold increases in σE activity compared to the activity of the wild-type strain (Fig. 5A). The σE activity was reduced to the basal level by inducing expression of the eps operon with the addition of arabinose to the medium (Fig. 5A). Similar results were observed in the single-gene-deletion mutant TRHΔepsL, in which there was an approximately fivefold greater stimulation of transcription (Fig. 5A). The elevated activity of rpoE in the TRHΔepsL mutant was restored to wild-type levels by ectopic expression of epsL (ΔepsL+pEpsL) (Fig. 5A). A twofold upregulation of rpoE also was observed for the PBAD::eps strain grown in the absence of arabinose in stationary-phase cells, but the increase was not statistically significant.
FIG. 5.
Increased σE activity in V. cholerae lacking functional T2S machinery. (A) The expression of the rpoEP2::lux fusion gene was determined with wild-type, PBAD::eps, ΔepsL, and ΔepsL+pEpsL strains of V. cholerae TRH7000 grown in LB broth at 37°C to mid-log and stationary phase (OD600 = 0.5 and OD600 = 4, respectively). The increased σE activities observed in PBAD::eps grown in the absence of arabinose (−) and TRHΔepsL strains could be complemented by the addition of 0.01% arabinose (+) to the growth medium and the expression of plasmid-encoded EpsL, respectively. The results presented are the means and corresponding SEM from three independent experiments. The difference in the induction of the σE response between the wild-type and PBAD::eps strains grown in the absence of arabinose and that of the TRHΔepsL mutant was statistically significant (P < 0.004) in mid-log-phase cultures, while the difference between stationary-phase cultures was not significant (P > 0.05). (B) Wild-type and rpoE mutant strains of V. cholerae O395 containing plasmid pMS43 were grown in LB broth at 37°C to late stationary phase (16 h) in the absence (−) or presence (+) of 10 μM IPTG to induce the expression of the dominant-negative mutant protein EpsE-K270A. Culture supernatants were separated from cells by centrifugation, matched by equivalent OD600 units, and analyzed by SDS-PAGE and silver staining.
As upregulation of σE activity by either overexpression of the rpoE gene or inactivation of the gene encoding the anti-σE factor RseA also can result in growth defects and compromised outer membrane integrity (50, 61), it was unclear whether inactivation of the eps genes causes membrane defects that in turn induce the σE stress response or, alternatively, whether the loss of Eps function induces σE expression, which in turn causes the outer membrane defects. In a first attempt to distinguish between these possibilities, we determined the effect of inactivating the Eps system on the outer membrane integrity of a V. cholerae rpoE mutant. This was accomplished by expression of the dominant-negative EpsE mutant, EpsE-K270A, in wild-type and rpoE mutant strains of V. cholerae O395 (49). If upregulation of rpoE is responsible for the increased outer membrane permeability, then expression of EpsE-K270A should not result in the accumulation of periplasmic proteins in the culture supernatant of the rpoE mutant. If, on the other hand, the σE activity is induced in response to the cell envelope damage caused by inactivation of eps genes, then the outer membrane in the rpoE mutant also should be compromised. Examination of supernatants isolated from late-stationary-phase cultures revealed that overproduction of EpsE-K270A resulted in increased leakage of intracellular proteins into the growth medium of both wild-type and rpoE mutant strains (Fig. 5B). Consistent with these findings, similar observations were made when EpsE-K270A was expressed in mutant strains lacking DegS or YeaL (49), in which σE remains permanently bound to the anti-σE factor RseA and cannot be activated (data not shown). Taken together, these results suggest that the σE activity is induced in response to the outer membrane damage caused by inactivation of the eps genes.
Suppression of growth defect and leaky outer membrane.
The eps mutants are not capable of growth in LB broth lacking NaCl (data not shown), while the wild-type strains grow without salt, albeit slowly, suggesting that eps mutants are sensitive to hypoosmotic growth conditions. To test this hypothesis and to determine if the growth defect can be suppressed by the addition of osmoprotectants, we compared the growth of the NΔeps mutant to that of its isogenic wild type, strain N16961, in the absence or presence of two osmolytes, sucrose and glucose. Addition of sucrose (Fig. 6A) or glucose (data not shown) to the growth medium decreased the NΔeps mutant doubling time from 90 to 25 min, comparable to the 20-min generation time of wild-type cells. Titration of the sugars (from 1 to 10% final concentration) revealed that the minimal concentration of glucose and sucrose that suppressed the growth defect in the mutant strain was 5% (data not shown). We next examined the protein profiles of the culture supernatants isolated from the wild-type, NΔeps mutant, and NΔeps+pEps strains following growth in the absence or presence of 5% glucose or sucrose. As mentioned before, a large amount of protein was detected in the culture supernatant of the NΔeps mutant grown in LB broth without glucose and sucrose (Fig. 6B). In contrast, considerably less protein was observed in the culture supernatants isolated from the NΔeps mutant grown in the presence of glucose or sucrose. The results presented in Fig. 6B suggest that these sugars prevent the nonspecific release of periplasmic content into the growth medium. The level of the periplasmic enzyme β-lactamase in the culture supernatant was reduced from 49% in LB broth alone to 29 and 25% in LB broth containing glucose and sucrose, respectively (Fig. 6C). In addition, the level of extracellular GFP was decreased from 6.0% ± 0.1% to 1.3% ± 0.3% and 0.7% ± 0.3% (means ± SEM) when the NΔeps mutant was grown in the presence of glucose and sucrose, respectively.
Although a slight enhancement of growth in the presence of bile salts on LB agar supplemented with sucrose was observed equally for the wild-type and NΔeps mutant strains, the mutant cells remained extremely sensitive to this detergent-like agent. While bile salts alone at a 0.05% final concentration resulted in 50% inhibition of growth of the wild-type strain (Table 2), the presence of sucrose improved the growth and resulted in only 23% growth inhibition (data not shown). Similarly, the level of growth inhibition by 0.0005% bile salts of the mutant strain in the absence and presence of sucrose were 50 and 12%, respectively. The NΔeps mutant still was not capable of growth on higher bile salts concentrations in the presence of sucrose, however, suggesting that the sugars do not restore the integrity of the outer membrane per se. Instead, these saccharides may protect the eps mutants, possibly by reestablishing the osmotic balance within the cells, thus preventing the leakage of periplasmic material. Further support for the suggestion that the sugars do not restore the outer membrane to wild-type function is the finding that the level of the outer membrane porin, OmpU, remained low in the NΔeps mutant following growth overnight in LB broth supplemented with sucrose or glucose (Fig. 7).
When additional sugars (lactose, galactose, sorbitol, melibiose, raffinose, xylose, and arabinose) or osmoprotectants (glycine-betaine and proline) were tested, only maltose and mannose were found to increase the growth rate of the NΔeps mutant and to prevent the release of periplasmic content (data not shown). The reason why the other saccharides tested were not capable of protecting the mutant cells is not known; however, none of these sugars is metabolized by V. cholerae (41).
DISCUSSION
Inactivation of the well-conserved T2S system, Eps, in V. cholerae results in pleiotropic effects. While the outer membrane is capable of supporting assembly and function of certain complex structures, such as the polar flagellum and the toxin-coregulated pilus (8, 14, 64; also data not shown), other properties of the outer membrane are severely compromised when the Eps system is inactivated. For example, the protein content of the outer membrane in eps mutants is altered such that the levels of OmpU, OmpT, OmpS, and perhaps also OmpV and OmpW are reduced compared to wild-type levels (64, 77). Inactivation of the T2S system also increases the sensitivity to agents normally excluded by the outer membrane, such as polymyxin B and bile salts (Table 2). Together, the outer membrane changes likely are responsible for the pronounced growth defect of the eps mutants. The outer membrane and secretion defects of the NΔeps mutant also contribute to the inability to survive the mouse gastrointestinal tract. This may be due to the loss of secretion of an important colonization factor and/or the increased sensitivity the mutant displays to bile salts and antimicrobial peptides, although increased sensitivity to bile salts by itself does not appear to affect the ability of V. cholerae to colonize the mouse infant intestine (64). Previously, pilD mutants of V. cholerae were shown to be defective in colonization of the infant mouse model. PilD is an enzyme that participates in many pathways, including processing of the pseudopilins of the T2S system as well as the type IV pilin subunits PilA, TcpA, and MshA (21, 47). Hence, the deficiency in colonization of the pilD mutants could not be attributed to the loss of function of a single pathway. Here we show that inactivation of the T2S system results in a phenotype similar to that of the pilD mutants, suggesting that the colonization defect of the pilD mutants is, in part, due to defects brought upon T2S disruption.
One of the consequences of the altered outer membrane in the eps mutants is the leakage of periplasmic material. Significant amounts of β-lactamase and AP escape from the periplasmic compartment to the culture medium during growth. Interestingly, while these resident periplasmic enzymes are not retained efficiently by the outer membrane, proteins normally transported by the Eps system do not appear to leak out from the eps mutants to the same degree. Using a combination of detection methods including immunoblotting (Fig. 2C) and enzyme-linked immunosorbent assay (13, 64, 77, 78), we and others have shown that neither cholera toxin nor EtxB leak out in appreciable amounts to the culture medium of eps mutants. If the extracellular protease and lipase do leak out, they must be inactive, as is the case for the LasB protease, which diffuses out of secretion-defective P. aeruginosa LPS mutants in an inactive form (53). The reason for the differential leakage in the V. cholerae eps mutants is not known. It is not dependent merely on size, as one of the released proteins, AP, is a dimer of 94 kDa, while a retained protein, the assembled EtxB pentamer, is only 58 kDa. Differences in shape or surface properties may be responsible for the selective release. Alternatively, the retained nonsecreted proteins may be trapped through interaction(s) with other cell envelope constituents in the eps mutants and therefore cannot diffuse freely into the culture medium.
The leaky phenotype is not due simply to decreased levels of the major porin OmpU or OmpT, as the structural integrity of the outer membrane remains unaffected in mutant strains lacking these porins (48, 64). The leakage could, however, be attributed to the combined loss of several outer membrane proteins. Consistent with this suggestion is the recent finding that inactivation of the E. coli ompR gene results in a reduced growth rate, a leaky outer membrane, and increased sensitivity to detergents (50). OmpR is a response regulator that controls the expression of several outer membrane proteins, including OmpF and OmpC (20). Similar pleiotropic effects also are associated with mutations of the Tol-Pal system in species such as E. coli, V. cholerae, and Erwinia chrysanthemi (4, 5, 45, 90). Besides providing a means for colicins and filamentous phages to enter bacterial cells, the Tol-Pal system also has been suggested to maintain the integrity/stability of the outer membrane (24) and to participate in cell division by assisting in the invagination of the outer membrane during septum formation (23). Other cell envelope mutants with phenotypes resembling the eps phenotype arise from mutations in genes encoding one of the most abundant outer membrane proteins, Lpp (86, 91), the Tat system (50, 84), or in genes coding for proteins involved in the biosynthesis of phospholipids and LPS (42).
The physiological phenotype of eps mutants suggests that the T2S complex plays a role in maintaining the integrity of the cell envelope, perhaps by supporting transport of a subset of outer membrane constituents. A recent transcriptome analysis of V. cholerae N16961 indicated that the expression of the eps genes is reduced in an rpoE mutant and increased in a mutant strain that produces an excess of active RpoE due to inactivation of rseA (16). Although these findings suggest that the eps genes are positively regulated by σE, further experimentation is needed to verify that σE directs the transcription of the eps gene cluster. Being part of the σE regulon would be a further indication of a role for the Eps system in V. cholerae outer membrane biogenesis.
Whatever the role of the Eps system in outer membrane biogenesis, the altered outer membrane architecture of the eps mutants may prevent proper insertion and assembly of outer membrane proteins, resulting in their misfolding and periplasmic accumulation (72). This may, in turn, result in induction of the σE stress response with a mechanism similar to that described for E. coli (reviewed in references 1 and 2). The induction of expression from the P2 promoter of the rpoE gene in our eps mutants is indicative of increased σE activity, consistent with our suggestion that a cell envelope stress response is induced when T2S is prevented. The increased σE activity likely upregulates the expression of other σE-dependent genes as well, and as some of these genes encode proteases, including the DegP homolog DO (16), which has been shown to eliminate misfolded proteins (56, 83), the reduced levels of some of the outer membrane proteins in the eps mutants may be due to proteolytic degradation.
When secretion via the T2S system was blocked by expression of the dominant-negative mutant protein EpsE-K270A, damage to the outer membrane was observed in both wild-type and rpoE mutant strains of V. cholerae. The rpoE mutant may be slightly more sensitive to EpsE-K270A expression than its parental wild-type strain, as more material appeared to leak out to the culture medium of this mutant (Fig. 5B). While this suggests that the σE regulon serves to protect the cells to some degree from the damage that occurs when the T2S system is inactivated, quantitative analysis of the level of leakiness has to await the construction of an Δeps ΔrpoE mutant, as the dominant-negative plasmid system used in this study is somewhat unstable. It is possible, however, that such a mutant is not viable.
The release of periplasmic proteins to the growth medium may be a consequence of hypoosmotic swelling of the eps mutant cells due to the altered outer membrane architecture. Less of the periplasmic material is released when the eps mutants are grown in medium containing sugar at a high concentration, suggesting that swelling no longer occurs. The reason only metabolizable sugars prevent the leakage is not clear, but it may be that the protection by these sugars is mediated within the bacterial cell. Transport of the sugars into the periplasmic compartment or perhaps even the cytoplasm may be a prerequisite for osmoprotection. It is possible that the normal protection against hypoosmotic conditions by osmoregulated periplasmic glucans (membrane-derived oligosaccharides) is compromised in the eps mutants (7, 9, 54) but can be compensated for by the import of saccharides such as glucose and sucrose.
Acknowledgments
We gratefully acknowledge Victor DiRita for help with the mouse infection studies and Tanya L. Johnson for critically reading the manuscript. We thank Karl Klose for OmpU antibodies, Jyl Matson and Victor DiRita for ΔrpoE, ΔdegS, and ΔyaeL mutant strains of V. cholerae O395, and Bonnie Bassler and Brian Hammer for plasmid pBBRlux.
This work was supported by grant AI49294 from the National Institutes of Health (to M.S.).
Footnotes
Published ahead of print on 21 September 2007.
REFERENCES
- 1.Ades, S. E. 2004. Control of the alternative sigma factor σE in Escherichia coli. Curr. Opin. Microbiol. 7:157-162. [DOI] [PubMed] [Google Scholar]
- 2.Alba, B. M., and C. A. Gross. 2004. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol. Microbiol. 52:613-619. [DOI] [PubMed] [Google Scholar]
- 3.Angelichio, M. J., J. Spector, M. K. Waldor, and A. Camilli. 1999. Vibrio cholerae intestinal population dynamics in the suckling mouse model of infection. Infect. Immun. 67:3733-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernadac, A., M. Gavioli, J. C. Lazzaroni, S. Raina, and R. Lloubes. 1998. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 180:4872-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bina, J. E., and J. J. Mekalanos. 2001. Vibrio cholerae tolC is required for bile resistance and colonization. Infect. Immun. 69:4681-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitter, W., R. van Boxtel, M. Groeneweg, P. S. Carballo, U. Zahringer, J. Tommassen, and M. Koster. 2007. Species-specific functioning of the Pseudomonas XcpQ secretin: role for the C-terminal homology domain and lipopolysaccharide. J. Bacteriol. 189:2967-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohin, J. P. 2000. Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiol. Lett. 186:11-19. [DOI] [PubMed] [Google Scholar]
- 8.Bose, N., S. M. Payne, and R. K. Taylor. 2002. Type 4 pilus biogenesis and type II-mediated protein secretion by Vibrio cholerae occur independently of the TonB-facilitated proton motive force. J. Bacteriol. 184:2305-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchart, F., A. Delangle, J. Lemoine, J. P. Bohin, and J. M. Lacroix. 2007. Proteomic analysis of a non-virulent mutant of the phytopathogenic bacterium Erwinia chrysanthemi deficient in osmoregulated periplasmic glucans: change in protein expression is not restricted to the envelope, but affects general metabolism. Microbiology 153:760-767. [DOI] [PubMed] [Google Scholar]
- 10.Camberg, J. L., T. L. Johnson, M. Patrick, J. Abendroth, W. G. Hol, and M. Sandkvist. 2007. Synergistic stimulation of EpsE ATP hydrolysis by EpsL and acidic phospholipids. EMBO J. 26:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 12.Cianciotto, N. P. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 13:581-588. [DOI] [PubMed] [Google Scholar]
- 13.Connell, T. D., D. J. Metzger, M. Wang, M. G. Jobling, and R. K. Holmes. 1995. Initial studies of the structural signal for extracellular transport of cholera toxin and other proteins recognized by Vibrio cholerae. Infect. Immun. 63:4091-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, B. M., E. H. Lawson, M. Sandkvist, A. Ali, S. Sozhamannan, and M. K. Waldor. 2000. Convergence of the secretory pathways for cholera toxin and the filamentous phage, CTXphi. Science 288:333-335. [DOI] [PubMed] [Google Scholar]
- 15.Davis, B. M., and M. K. Waldor. 2000. CTXphi contains a hybrid genome derived from tandemly integrated elements. Proc. Natl. Acad. Sci. USA 97:8572-8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding, Y., B. M. Davis, and M. K. Waldor. 2004. Hfq is essential for Vibrio cholerae virulence and downregulates sigma expression. Mol. Microbiol. 53:345-354. [DOI] [PubMed] [Google Scholar]
- 17.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drew, D., D. Sjostrand, J. Nilsson, T. Urbig, C. N. Chin, J. W. de Gier, and G. von Heijne. 2002. Rapid topology mapping of Escherichia coli inner-membrane proteins by prediction and PhoA/GFP fusion analysis. Proc. Natl. Acad. Sci. USA 99:2690-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duguay, A. R., and T. J. Silhavy. 2004. Quality control in the bacterial periplasm. Biochim. Biophys. Acta 1694:121-134. [DOI] [PubMed] [Google Scholar]
- 20.Forst, S., J. Delgado, G. Ramakrishnan, and M. Inouye. 1988. Regulation of ompC and ompF expression in Escherichia coli in the absence of envZ. J. Bacteriol. 170:5080-5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fullner, K. J., and J. J. Mekalanos. 1999. Genetic characterization of a new type IV-A pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect. Immun. 67:1393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garen, A., and C. Levinthal. 1960. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim. Biophys. Acta 38:470-483. [DOI] [PubMed] [Google Scholar]
- 23.Gerding, M. A., Y. Ogata, N. D. Pecora, H. Niki, and P. A. de Boer. 2007. The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Mol. Microbiol. 63:1008-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goemaere, E. L., E. Cascales, and R. Lloubes. 2007. Mutational analyses define helix organization and key residues of a bacterial membrane energy-transducing complex. J. Mol. Biol. 366:1424-1436. [DOI] [PubMed] [Google Scholar]
- 25.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock, R. E. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 27.Herrington, D. A., R. H. Hall, G. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirst, T. R., and J. Holmgren. 1987. Transient entry of enterotoxin subunits into the periplasm occurs during their secretion from Vibrio cholerae. J. Bacteriol. 169:1037-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirst, T. R., L. L. Randall, and S. J. Hardy. 1984. Cellular location of heat-labile enterotoxin in Escherichia coli. J. Bacteriol. 157:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirst, T. R., J. Sanchez, J. B. Kaper, S. J. Hardy, and J. Holmgren. 1984. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc. Natl. Acad. Sci. USA 81:7752-7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman, C. S., and A. Wright. 1985. Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc. Natl. Acad. Sci. USA 82:5107-5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes, R. K., M. L. Vasil, and R. A. Finkelstein. 1975. Studies on toxinogenesis in Vibrio cholerae. III. Characterization of nontoxinogenic mutants in vitro and in experimental animals. J. Clin. Investig. 55:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard, S. P., J. Critch, and A. Bedi. 1993. Isolation and analysis of eight exe genes and their involvement in extracellular protein secretion and outer membrane assembly in Aeromonas hydrophila. J. Bacteriol. 175:6695-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howard, S. P., C. Gebhart, G. R. Langen, G. Li, and T. G. Strozen. 2006. Interactions between peptidoglycan and the ExeAB complex during assembly of the type II secretin of Aeromonas hydrophila. Mol. Microbiol. 59:1062-1072. [DOI] [PubMed] [Google Scholar]
- 35.Ichige, A., K. Oishi, and S. Mizushima. 1988. Isolation and characterization of mutants of a marine Vibrio strain that are defective in production of extracellular proteins. J. Bacteriol. 170:3537-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jalajakumari, M. B., and P. A. Manning. 1990. Nucleotide sequence of the gene, ompW, encoding a 22kDa immunogenic outer membrane protein of Vibrio cholerae. Nucleic Acids Res. 18:2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang, B., and S. P. Howard. 1992. The Aeromonas hydrophila exeE gene, required both for protein secretion and normal outer membrane biogenesis, is a member of a general secretion pathway. Mol. Microbiol. 6:1351-1361. [DOI] [PubMed] [Google Scholar]
- 38.Johnson, T. L., J. Abendroth, W. G. Hol, and M. Sandkvist. 2006. Type II secretion: from structure to function. FEMS Microbiol. Lett. 255:175-186. [DOI] [PubMed] [Google Scholar]
- 39.Judson, N., and J. J. Mekalanos. 2000. Transposon-based approaches to identify essential bacterial genes. Trends Microbiol. 8:521-526. [DOI] [PubMed] [Google Scholar]
- 40.Kagami, Y., M. Ratliff, M. Surber, A. Martinez, and D. N. Nunn. 1998. Type II protein secretion by Pseudomonas aeruginosa: genetic suppression of a conditional mutation in the pilin-like component XcpT by the cytoplasmic component XcpR. Mol. Microbiol. 27:221-233. [DOI] [PubMed] [Google Scholar]
- 41.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kloser, A., M. Laird, M. Deng, and R. Misra. 1998. Modulations in lipid A and phospholipid biosynthesis pathways influence outer membrane protein assembly in Escherichia coli K-12. Mol. Microbiol. 27:1003-1008. [DOI] [PubMed] [Google Scholar]
- 43.Kostakioti, M., C. L. Newman, D. G. Thanassi, and C. Stathopoulos. 2005. Mechanisms of protein export across the bacterial outer membrane. J. Bacteriol. 187:4306-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovacikova, G., and K. Skorupski. 2002. The alternative sigma factor σE plays an important role in intestinal survival and virulence in Vibrio cholerae. Infect. Immun. 70:5355-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazzaroni, J. C., N. Fognini-Lefebvre, and R. Portalier. 1989. Cloning of the excC and excD genes involved in the release of periplasmic proteins by Escherichia coli K12. Mol. Gen. Genet. 218:460-464. [DOI] [PubMed] [Google Scholar]
- 46.Lenz, D. H., K. C. Mok, B. N. Lilley, R. V. Kulkarni, N. S. Wingreen, and B. L. Bassler. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118:69-82. [DOI] [PubMed] [Google Scholar]
- 47.Marsh, J. W., and R. K. Taylor. 1998. Identification of the Vibrio cholerae type 4 prepilin peptidase required for cholera toxin secretion and pilus formation. Mol. Microbiol. 29:1481-1492. [DOI] [PubMed] [Google Scholar]
- 48.Mathur, J., and M. K. Waldor. 2004. The Vibrio cholerae ToxR-regulated porin OmpU confers resistance to antimicrobial peptides. Infect. Immun. 72:3577-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matson, J. S., and V. J. DiRita. 2005. Degradation of the membrane-localized virulence activator TcpP by the YaeL protease in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 102:16403-16408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McBroom, A. J., A. P. Johnson, S. Vemulapalli, and M. J. Kuehn. 2006. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 188:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ménard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meselson, M., and R. Yuan. 1968. DNA restriction enzyme from E. coli. Nature 217:1110-1114. [DOI] [PubMed] [Google Scholar]
- 53.Michel, G., G. Ball, J. B. Goldberg, and A. Lazdunski. 2000. Alteration of the lipopolysaccharide structure affects the functioning of the Xcp secretory system in Pseudomonas aeruginosa. J. Bacteriol. 182:696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller, K. J., E. P. Kennedy, and V. N. Reinhold. 1986. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science 231:48-51. [DOI] [PubMed] [Google Scholar]
- 55.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Misra, R., A. Peterson, T. Ferenci, and T. J. Silhavy. 1991. A genetic approach for analyzing the pathway of LamB assembly into the outer membrane of Escherichia coli. J. Biol. Chem. 266:13592-13597. [PubMed] [Google Scholar]
- 57.Morales, V. M., A. Backman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-47. [DOI] [PubMed] [Google Scholar]
- 58.Nandi, B., R. K. Nandy, A. Sarkar, and A. C. Ghose. 2005. Structural features, properties and regulation of the outer-membrane protein W (OmpW) of Vibrio cholerae. Microbiology 151:2975-2986. [DOI] [PubMed] [Google Scholar]
- 59.Newman, J. R., and C. Fuqua. 1999. Broad-host-range expression vectors that carry the L-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227:197-203. [DOI] [PubMed] [Google Scholar]
- 60.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nitta, T., H. Nagamitsu, M. Murata, H. Izu, and M. Yamada. 2000. Function of the σE regulon in dead-cell lysis in stationary-phase Escherichia coli. J. Bacteriol. 182:5231-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Overbye, L. J., M. Sandkvist, and M. Bagdasarian. 1993. Genes required for extracellular secretion of enterotoxin are clustered in Vibrio cholerae. Gene 132:101-106. [DOI] [PubMed] [Google Scholar]
- 63.Peek, J. A., and R. K. Taylor. 1992. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 89:6210-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Provenzano, D., C. M. Lauriano, and K. E. Klose. 2001. Characterization of the role of the ToxR-modulated outer membrane porins OmpU and OmpT in Vibrio cholerae virulence. J. Bacteriol. 183:3652-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Provenzano, D., D. A. Schuhmacher, J. L. Barker, and K. E. Klose. 2000. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect. Immun. 68:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pukatzki, S., A. T. Ma, D. Sturtevant, B. Krastins, D. Sarracino, W. C. Nelson, J. F. Heidelberg, and J. J. Mekalanos. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA 103:1528-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raina, S., D. Missiakas, and C. Georgopoulos. 1995. The rpoE gene encoding the σE (σ24) heat shock sigma factor of Escherichia coli. EMBO J. 14:1043-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rouvière, P. E., A. De Las Peñas, J. Mecsas, C. Z. Lu, K. E. Rudd, and C. A. Gross. 1995. rpoE, the gene encoding the second heat-shock sigma factor, σE, in Escherichia coli. EMBO J. 14:1032-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roy, N. K., R. K. Ghosh, and J. Das. 1982. Monomeric alkaline phosphatase of Vibrio cholerae. J. Bacteriol. 150:1033-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rudd, K. E., H. J. Sofia, E. V. Koonin, G. Plunkett III, S. Lazar, and P. E. Rouviëre. 1995. A new family of peptidyl-prolyl isomerases. Trends Biochem. Sci. 20:12-14. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz, N., D. Kahne, and T. J. Silhavy. 2006. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 4:57-66. [DOI] [PubMed] [Google Scholar]
- 73.Ruiz, N., and T. J. Silhavy. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122-126. [DOI] [PubMed] [Google Scholar]
- 74.Sandkvist, M. 2001. Type II secretion and pathogenesis. Infect. Immun. 69:3523-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sandkvist, M., M. Bagdasarian, S. P. Howard, and V. J. DiRita. 1995. Interaction between the autokinase EpsE and EpsL in the cytoplasmic membrane is required for extracellular secretion in Vibrio cholerae. EMBO J. 14:1664-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sandkvist, M., T. R. Hirst, and M. Bagdasarian. 1990. Minimal deletion of amino acids from the carboxyl terminus of the B subunit of heat-labile enterotoxin causes defects in its assembly and release from the cytoplasmic membrane of Escherichia coli. J. Biol. Chem. 265:15239-15244. [PubMed] [Google Scholar]
- 77.Sandkvist, M., L. O. Michel, L. P. Hough, V. M. Morales, M. Bagdasarian, M. Koomey, and V. J. DiRita. 1997. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J. Bacteriol. 179:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandkvist, M., V. Morales, and M. Bagdasarian. 1993. A protein required for secretion of cholera toxin through the outer membrane of Vibrio cholerae. Gene 123:81-86. [DOI] [PubMed] [Google Scholar]
- 79.Schäfer, A., A. Tauch, W. Jager, J. Kalinowski, G. Thierbach, and A. Puhler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 80.Scott, M. E., Z. Y. Dossani, and M. Sandkvist. 2001. Directed polar secretion of protease from single cells of Vibrio cholerae via the type II secretion pathway. Proc. Natl. Acad. Sci. USA 98:13978-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology 1:784-790. [Google Scholar]
- 82.Söderberg, M. A., O. Rossier, and N. P. Cianciotto. 2004. The type II protein secretion system of Legionella pneumophila promotes growth at low temperatures. J. Bacteriol. 186:3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spiess, C., A. Beil, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339-347. [DOI] [PubMed] [Google Scholar]
- 84.Stanley, N. R., K. Findlay, B. C. Berks, and T. Palmer. 2001. Escherichia coli strains blocked in Tat-dependent protein export exhibit pleiotropic defects in the cell envelope. J. Bacteriol. 183:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stevenson, G., D. I. Leavesley, C. A. Lagnado, M. W. Heuzenroeder, and P. A. Manning. 1985. Purification of the 25-kDa Vibrio cholerae major outer-membrane protein and the molecular cloning of its gene: ompV. Eur. J. Biochem. 148:385-390. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki, H., Y. Nishimura, S. Yasuda, A. Nishimura, M. Yamada, and Y. Hirota. 1978. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol. Gen. Genet. 167:1-9. [DOI] [PubMed] [Google Scholar]
- 87.Taylor, D. N., K. P. Killeen, D. C. Hack, J. R. Kenner, T. S. Coster, D. T. Beattie, J. Ezzell, T. Hyman, A. Trofa, M. H. Sjogren, et al. 1994. Development of a live, oral, attenuated vaccine against El Tor cholera. J. Infect. Dis. 170:1518-1523. [DOI] [PubMed] [Google Scholar]
- 88.Taylor, R. K., C. Manoil, and J. J. Mekalanos. 1989. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J. Bacteriol. 171:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Voulhoux, R., G. Ball, B. Ize, M. L. Vasil, A. Lazdunski, L. F. Wu, and A. Filloux. 2001. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 20:6735-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Webster, R. E. 1991. The tol gene products and the import of macromolecules into Escherichia coli. Mol. Microbiol. 5:1005-1011. [DOI] [PubMed] [Google Scholar]
- 91.Yem, D. W., and H. C. Wu. 1978. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J. Bacteriol. 133:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]