Abstract
The herpes simplex virus type 1 (HSV-1) neurovirulence gene encoding ICP34.5 controls the autophagy pathway. HSV-1 strains lacking ICP34.5 are attenuated in growth and pathogenesis in animal models and in primary cultured cells. While this growth defect has been attributed to the inability of an ICP34.5-null virus to counteract the induction of translational arrest through the PKR antiviral pathway, the role of autophagy in the regulation of HSV-1 replication is unknown. Here we show that HSV-1 infection induces autophagy in primary murine embryonic fibroblasts and that autophagosome formation is increased to a greater extent following infection with an ICP34.5-deficient virus. Elimination of the autophagic pathway did not significantly alter the replication of wild-type HSV-1 or ICP34.5 mutants. The phosphorylation state of eIF2α and viral protein accumulation were unchanged in HSV-1-infected cells unable to undergo autophagy. These data show that while ICP34.5 regulates autophagy, it is the prevention of translational arrest by ICP34.5 rather than its control of autophagy that is the pivotal determinant of efficient HSV-1 replication in primary cell culture.
To establish productive infections, viruses have a number of genes which inhibit the establishment of the host antiviral state and subvert host metabolic pathways to facilitate and amplify their own replication. Innate defenses of the host are induced by interferons (IFNs) which facilitate the establishment of the antiviral state, thereby suppressing viral replication and promoting cell survival (16). A major effect of the IFN response is to block translation and production of viral proteins (57). This translational arrest is largely achieved through activation of the double-stranded RNA-dependent protein kinase R (PKR) whose gene expression is up-regulated by type I IFNs (24, 32, 49). The translation initiation factor eIF2α is a major substrate of activated PKR, and once phosphorylated, eIF2α is unable to participate in the guanine nucleotide exchange reaction required for start codon recognition (48). This enables the host to slow virus infections, prevent spread, and promote overall survival. Not surprisingly, a wide variety of viruses have evolved strategies to counteract this shutoff of protein synthesis (reviewed in reference 13).
Herpes simplex virus type 1 (HSV-1) encodes several gene products that maintain a cellular environment favorable for virus replication. HSV-1 infection activates PKR and an antiviral response, and a major antagonist of this antiviral response is the HSV-1-encoded protein ICP34.5 (8). ICP34.5 acts throughout the viral life cycle, and one of its functions is to interact with protein phosphatase 1α (PP1α) via its C terminus (1, 44). This interaction redirects PP1α to dephosphorylate eIF2α, preventing the accumulation of the phosphorylated form (1, 12). ICP34.5 is critical for efficient replication, pathogenesis, and neurovirulence of HSV-1 in humans and animal models (5, 6, 45). The defects in growth and virulence of ICP34.5 null viruses are restored in type I IFN receptor- and PKR-deficient mice, indicating that ICP34.5 specifically targets these antiviral pathways (25, 26).
IFN and PKR have many effects on cellular metabolic pathways, including the process of autophagy (7, 18, 31, 52, 53, 55). Autophagy is a constitutive cellular process in which cytoplasmic components are sequestered and degraded by the lysosome to generate metabolic precursors, to remove damaged organelles and altered intracellular components, and to enhance or inhibit the replication of invading pathogens (37; reviewed in references 9 and 30). This process is induced by stress stimuli including starvation, growth factor deprivation, drug treatment, or infection. Following an induction stimulus, a crescent-shaped double-layered isolation membrane forms and then elongates and closes to generate the double-membraned autophagosome. The autophagosome fuses with the lysosome, and its contents and inner membrane are degraded by lysosomal enzymes. Formation of the autophagosome requires a conjugate of Atg5-Atg12, as well as a second conjugation system in which LC3-I and LC3-II are produced from the cleavage of LC3 (33, 35, 36, 54). Beclin 1 is also required for preautophagosome formation and is found in a complex with Vps34, a class III phosphoinositide 3-kinase, at the trans-Golgi network (21). This complex may supply phosphatidylinositol 3-phosphates to preautophagosome membranes, thereby facilitating the localization of Atg proteins (50).
Both PKR and phosphorylated eIF2α have been shown to promote autophagy, although the mechanism by which this occurs is unclear (51, 52). Gcn2, another eIF2α kinase, is required for starvation-induced autophagy in yeast, and PKR can functionally substitute for GCN2 in GCN2-disrupted yeast (51). Phosphorylation of eIF2α is necessary for effective translation of specific mRNAs, including the transcriptional activator Gcn4 (11). In yeast, a number of components of the autophagy pathway are targets of Gcn4 (41). Thus, phosphorylation of eIF2α resulting from starvation or viral infection may lead to the initiation of autophagy and other cellular programs to promote cell survival following diverse stress stimuli.
Autophagy is a component of the host defense response to a number of intracellular bacteria including Mycobacterium tuberculosis, group A Streptococcus, Shigella, and Salmonella, as well as the parasite Toxoplasma gondii (2, 4, 10, 28, 39, 42). Autophagy also has been shown to be antiviral against Sindbis virus, tobacco mosaic virus, and the DNA virus parvovirus B19 (27, 29, 40). In contrast, certain RNA viruses, such as coronaviruses, picornaviruses, murine hepatitis virus, and equine arterivirus, utilize the autophagosomal machinery to facilitate the assembly of RNA replication complexes (17, 46).
Consistent with these other pathogens, HSV-1 is also able to regulate autophagy, through the actions of ICP34.5. This regulation is believed to be achieved through the two known functions of ICP34.5, first through dephosphorylation of eIF2α and second through its ability to bind Beclin 1 (43, 51, 52). Murine embryonic fibroblasts (MEFs) infected with an ICP34.5-null virus have increased long-lived protein degradation, higher autophagic vacuole volume density, and increased numbers of virions per autophagosome compared to wild-type-infected cells (51, 52). This increase in autophagy is in part due to the inability of ICP34.5 to bind Beclin 1, since deletion of the ICP34.5 Beclin 1 binding domain renders HSV-1 unable to regulate autophagosome formation (43). This regulation of autophagy is critical for HSV-1 pathogenesis, as viruses lacking the Beclin 1 binding domain are severely neuroattenuated (43).
Taken together, these previous data suggested that the severe growth defect of an ICP34.5-deficient virus in cell culture and in vivo may not solely be due to its inability counteract translational arrest. Thus, failure to inhibit the autophagic pathway must also be considered. In this study, MEFs generated from atg5−/− and control mice provided a genetic system in which to address the impact of autophagy upon HSV-1 replication (34). Autophagy was induced upon viral infection but did not have a major effect upon viral replication in these permissive cells. This is consistent with a recent study demonstrating that HSV-1 (strain KOS) replication was similar in atg5−/− and wild-type MEFs (19). HSV-1-mediated dephosphorylation of eIF2α occurred normally in the absence of autophagy, and protein accumulation was similar in the presence and absence of atg5. This suggested that the lack of accumulation of viral proteins during HSV-1 infection was likely due to decreased protein synthesis as a result of phosphorylated eIF2α, as opposed to increased protein degradation due to autophagy. Additionally, the replication of the ICP34.5 Beclin 1 binding mutant was not impacted by the absence of autophagy. Since this mutant is able to block translational arrest, the prevention of translational arrest is a more critical function for ICP34.5 than the prevention of autophagy for maintaining HSV-1 replication. Regulation of autophagy by HSV-1, therefore, despite its proven pivotal impact on neurovirulence, does not impact similarly upon viral replication in permissive cultured cells.
MATERIALS AND METHODS
Cells, viruses, and growth assays.
Heterozygous atg5 mutant mice were interbred to obtain homozygous mutant embryos as previously described (23, 34). Primary MEFs were prepared from day 15 embryos in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum, 1× nonessential and essential amino acids, 2 mM L-glutamine, and penicillin-streptomycin. Embryos were screened individually via PCR for the targeted disruption of the atg5 gene. Once the MEFs were contact inhibited (about 48 h postplating), they were passaged two or three times prior to infection. The viruses used in this study, 17termA and its corresponding marker-rescued virus 17termAR, were kindly provided by Richard Thompson, University of Cincinnati, Cincinnati, OH. For growth curve determination, primary MEFs were infected with the appropriate virus at a multiplicity of 0.01 PFU/cell. The virus was allowed to adsorb for 1 h, a viral inoculum was removed, and fresh medium was added. At various times postinfection, cells and supernatants were harvested and freeze-thawed and titers were determined by plaque assay on Vero cells.
To generate the Beclin 1 binding mutant (Δ68H), a plasmid containing ICP34.5 lacking amino acids 68 to 87, pDA07 (43), was cotransfected into Vero cells with infectious viral DNA from HSV-1 strain 17syn+ by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Virus from this transfection was plaque purified and screened for the incorporation of the deletion by PCR with the primers 5′-GCACATGCTTGCCTGTCAAACTCT-3′ and 5′-TGTAACGTTAGACCGAGTTCGCCG-3′. These primers generate a PCR product that spans the deletion, which eliminates a HinfI site. Wild-type and Δ68H templates generate 808-bp and 746-bp PCR products, respectively. Following digestion with HinfI, wild-type PCR products were cut into 542- and 265-bp fragments but Δ68H DNA was uncut. Plaques lacking the HinfI site were plaque purified three times prior to isolation of viral DNA and Southern blot analysis. The marker-rescued virus (Δ68HR) was generated by cotransfection of Δ68H infectious viral DNA with pDA04 (43) and isolated as described above for the Δ68H mutant. Southern blot analysis of viral DNA was performed as previously described (47). Following DNA digestion of viral stocks with HinfI, an 812-bp BspEI/NcoI fragment corresponding to nucleotides 511 to 1323 of the HSV-1 genome was labeled with [32P]dCTP by random priming. This fragment was used as a probe to confirm the presence or absence of nucleotides encoding amino acids 68 to 87 of ICP34.5 in the bacterial artificial chromosome (BAC)-derived mutant and marker-rescued viruses.
Western blot analyses.
Primary atg5−/− and atg5+/+ MEFs were mock infected or infected with 17termAR or 17termA at a multiplicity of 5 PFU/cell for 15 h. Medium was removed, cells were rinsed with cold phosphate-buffered saline, and lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail set III [Calbiochem]) was added. Whole-cell lysates (30 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with a polyclonal antibody to Atg5 (SO4) (34), LC3 (20), ICP34.5 (38), phosphorylated eIF2α (BioSource, Camarillo, CA), or total eIF2α (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins were detected with an ECL-Plus kit (Amersham Pharmacia, Piscataway, NJ). To visualize proteins, blots were scanned on a Molecular Dynamics STORM 860 PhosphorImager.
Metabolic labeling.
Primary atg5−/− and atg5+/+ MEFs were mock infected or infected with 17termAR or 17termA at a multiplicity of 5 PFU/cell for 6 or 12 h. At this time, medium was removed and cells were washed twice with DMEM lacking cysteine and methionine and incubated with cysteine and methionine-free DMEM containing 50 μCi/ml 35S-labeled cysteine and methionine (Tran35S-label; MP Biomedicals, Irvine, CA) for 2 h. Whole-cell extracts were harvested, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and exposed for autoradiography.
Transmission electron microscopy.
For ultrastructural analysis, primary atg5+/+ MEFs were mock infected or infected with 17termAR or 17termA at a multiplicity of 5 PFU/cell for 12 h. Cells were fixed in 2% paraformaldehyde-2.5% glutaraldehyde (Polysciences Inc., Warrington, PA) in 100 mM phosphate buffer, pH 7.2, for 1 h at room temperature. Following three washes in phosphate buffer, cells were postfixed in 1% osmium tetroxide (Polysciences Inc.) for 1 h at room temperature. The samples were then rinsed extensively in distilled H2O prior to en bloc staining with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, CA) for 1 h at room temperature. Following several rinses in distilled H2O, cells were dehydrated in a graded series of ethanol and embedded in Eponate 12 resin (Ted Pella Inc.). Sections of 70 to 80 nm were cut, stained with uranyl acetate and lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA, Inc., Peabody, MA). Approximately 25 cells were counted, and for each cell the number of autophagosomes and the number of virions within autophagosomes were examined. Autophagosomes were defined as double-membraned vacuoles measuring 0.3 to 2.0 μm with clearly recognizable cytoplasmic contents.
RESULTS
Autophagosome formation and virion degradation are increased in 17termA-infected MEFs.
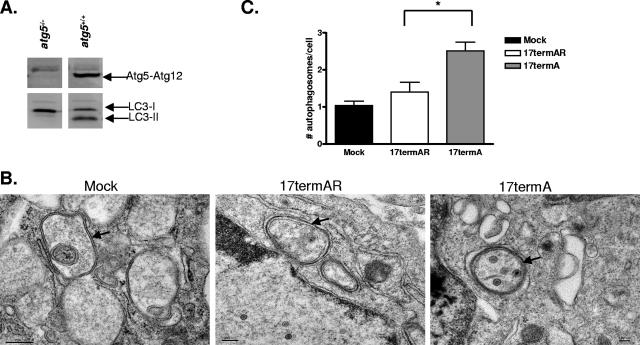
Previous studies indicated that 17termA-infected cells have increased levels of autophagy compared to 17termAR-infected cells, although differing cell types and methodologies for measuring autophagy have been used (43, 51, 55). We needed, therefore, to reconfirm that the expected level of autophagosome formation could be measured in our cultured MEFs. We showed that atg5−/− MEFs generated from atg5−/− embryos did not express the Atg5-Atg12 conjugate required for elongation of the autophagosomal membrane (Fig. 1A). Furthermore, LC3-II was absent in atg5−/− MEFs yet present in MEFs derived from atg5+/+ littermates under nutrient rich conditions, demonstrating that the atg5−/− cells were autophagy defective (Fig. 1A).
FIG. 1.
ICP34.5 partially controls HSV-1-induced autophagosome formation and engulfment of virions. (A) Western blot analysis of lysates harvested from atg5−/− and atg5+/+ MEFs probed with polyclonal antibodies to Atg5 (SO4) and LC3. The majority of Atg5 was conjugated to Atg12 in the cytoplasm. (B) Representative electron micrographs of mock-, 17termAR-, and 17termA-infected Atg5+/+ MEFs. Arrows indicate representative autophagosomes that would be scored positive in panel C. Scale bars, 200 nm (left part) and 100 nm (middle and right parts). (C) Quantitation of the numbers of autophagosomes in mock-, 17termAR-, and 17termA-infected Atg5+/+ MEFs. Results shown represent data collected from three independent experiments. Data shown represent the mean number of autophagosomes per cell per condition ± the standard error of the mean. An asterisk indicates P < 0.05 by Student's t test.
atg5+/+ MEFs were mock infected or infected with 17termAR or 17termA at a multiplicity of 5 PFU/cell. At 12 h postinfection, cells were fixed and examined by electron microscopy and autophagosomes were identified by their characteristic double-membrane, size of 0.3 to 2.0 μm, and engulfed cytoplasmic components. Some autophagosomes were present in mock-, 17termAR- and 17termA-infected MEFs (Fig. 1B). In MEFs infected with 17termAR, however, autophagosomes appeared to have different morphological characteristics in that they were larger and the membranes were less electron dense (Fig. 1A, middle part). In addition, 17termA-infected cells had smaller, more clearly defined autophagosomes containing numerous HSV-1 virions (Fig. 1B, right part). Most importantly, infection with 17termA resulted in significantly more autophagosomes than with 17termAR (Fig. 1C). HSV-1-infected MEFs contained slightly more autophagosomes than mock-infected cells, but this difference was not significant. Additionally, only 3% of the autophagosomes in 17termAR-infected cells contained virions while 15% of the autophagosomes in cells infected with 17termA contained virions. These data suggest that ICP34.5 regulates autophagy induced by HSV-1 infection.
HSV-1 replication is increased in cells deficient in atg5 independent of ICP34.5 function.
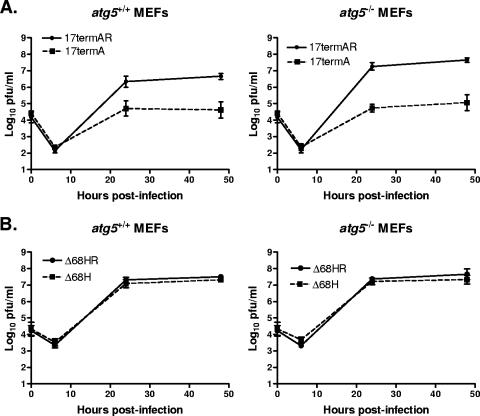
The replication of viruses lacking ICP34.5 was restored to wild-type levels in MEFs lacking PKR or expressing a nonphosphorylatable allele of eIF2α (eIF2α S51A) (52, 56). Additionally, the attenuation of an ICP34.5-null virus was restored to full virulence in mice lacking PKR (26). PKR activity and phosphorylation of eIF2α are required not only for induction of autophagy but also for induction of translational arrest following HSV-1 infection. Autophagy and translational arrest are both blocked by ICP34.5, but the following question remained: is blockage of both functions important for maintaining wild-type growth? To answer this question, MEFs unable to undergo autophagy were generated from atg5−/− mice. The growth of the ICP34.5-null virus (17termA), as well as its marker-rescued virus (17termAR), was analyzed in these cells.
At 24 and 48 h postinfection at a low multiplicity, replication of 17termAR was modestly increased in atg5−/− MEFs compared to that in atg5+/+ MEFs (Fig. 2A). The growth of wild-type strain 17 was comparable to that of 17termAR (data not shown). The replication of 17termA was unchanged, remaining decreased 100-fold relative to 17termAR in both atg5−/− and atg5+/+ cells (Fig. 2A). It is possible, however, that translational arrest following 17termA infection was preventing effects of autophagy from being observed. In order to rule out this possibility, we assessed the growth of an ICP34.5 mutant lacking amino acids 68 to 87. This virus is unable to bind the autophagy-promoting protein Beclin 1 but still mediates eIF2α dephosphorylation, thereby enabling translation to proceed (43).
FIG. 2.
Autophagy does not significantly affect HSV-1 growth. Primary Atg5−/− and Atg5+/+ MEFs were infected at a multiplicity of 0.01 PFU/cell. Cells and supernatants were collected at the indicated times postinfection, and titers were determined on Vero cells. Results shown represent data collected from three independent experiments. Data points represent the geometric mean number of PFU/ml of material in which titers were determined ± the standard error of the mean for three samples per virus per time point. The growth of 17termAR was significantly different (P < 0.05 by Student's t test) at 48 h postinfection. The limit of detection of this assay, as indicated on the y axis, is 10 PFU/ml.
The previously described mutant and marker-rescued virus were generated via BAC-mediated mutagenesis, and these BAC-generated viruses were attenuated compared to wild-type strain 17 (unpublished results). Their phenotypes, however, with respect to ICP34.5 and autophagy remained true to their intended genotypes (43). Additionally, PCR analysis revealed that both BAC-generated viruses had mutations in oriL (unpublished results) which in other viruses have been shown to reduce virulence (3, 58). Therefore, we remade both the ICP34.5 Beclin 1 binding mutant (referred to as Δ68H) and its corresponding marker-rescued virus (referred to as Δ68HR) via homologous recombination. The sequences of these viruses were confirmed by PCR and Southern blotting, and both express ICP34.5 of the predicted size (data not shown). Both Δ68H and Δ68HR are able to mediate dephosphorylation of eIF2α, similar to wild-type HSV-1 (data not shown).
Similar to the growth phenotypes of 17termAR and 17termA, both Δ68H and Δ68HR grew comparably in atg5−/− and atg5+/+ MEFs (Fig. 2B). The replication of the BAC-derived mutants HSV-1 34.5Δ68-87 and HSV-1 34.5Δ68-87R was virtually indistinguishable in both atg5−/− and atg5+/+ MEFs (data not shown). These observations suggest that the severe growth defect of an ICP34.5-null virus was therefore not a result of its inability to counteract autophagy.
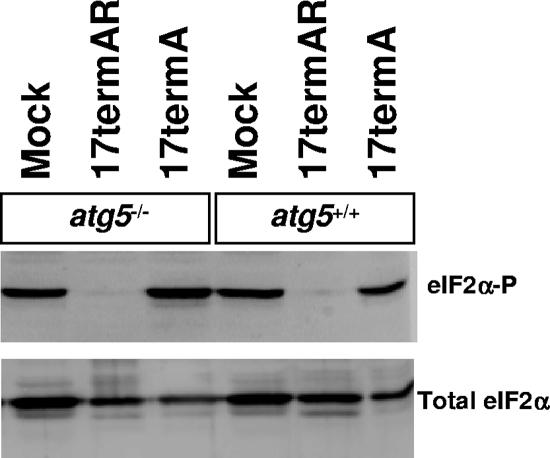
The phosphorylation state of eIF2α remains unchanged in the absence of autophagy.
The lack of ICP34.5 in 17termA results in decreased replication in MEFs, but it is unknown whether this is due to its inability to dephosphorylate eIF2α or loss of some other function, such as binding to Beclin 1 and subsequent inhibition of autophagy. A relevant observation is that LC3-II was not observed in MEFs expressing a nonphosphorylatable allele of eIF2α, indicating that phosphorylation of eIF2α is upstream of LC3 conversion and autophagosome formation (22). It was of interest, therefore, to determine the phosphorylation state of eIF2α following infection of autophagy-competent and -deficient cells. By using phosphospecific antibodies, we compared the levels of phosphorylated eIF2α in atg5−/− and atg5+/+ MEFs following HSV-1 infection. High levels of phosphorylated eIF2α were observed in mock-infected cells (Fig. 3). Mock treatment, so-called “mockulum,” was performed in the same fashion as viral infection with extracts of uninfected Vero cells. This control was included because infection procedures induce stress signaling pathways leading to the accumulation of phosphorylated eIF2α even in the absence of virus infection. Consistent with the severe growth defect of 17termA in both atg5+/+ and atg5−/− cells, the ICP34.5-null mutant was unable to direct dephosphorylation of eIF2α, regardless of the presence of atg5 (Fig. 3). In contrast, eIF2α was dephosphorylated in all MEFs infected with 17termAR (Fig. 3). Thus, as expected, autophagy levels in HSV-1 infected cells correlate with eIF2α phosphorylation.
FIG. 3.
The phosphorylation state of eIF2α is not affected by the autophagic pathway. Shown is a Western blot analysis of lysates of primary Atg5−/− and Atg5+/+ MEFs mock infected or infected with 17termAR or 17termA at a multiplicity of 5 PFU/cell for 15 h. Immunoblots were probed with polyclonal antibodies specific for eIF2α phosphorylated on Ser51 and total eIF2α.
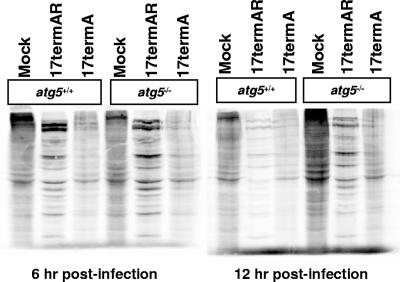
Autophagy has minimal impact upon viral protein accumulation.
Consistent with the ability of ICP34.5 to direct dephosphorylation of eIF2α, it has been shown that viral protein synthesis was significantly reduced in MEFs infected with an ICP34.5-null virus (52). While this phenotype has been attributed to a decrease in translation, an increase in autophagy-mediated protein degradation could also contribute to a decrease in viral protein accumulation. To distinguish between these possibilities, at 6 and 12 h postinfection, autophagy-competent and -deficient MEFs were metabolically labeled for 2 h to determine the levels of protein synthesis and accumulation. Given the relatively long labeling time for these experiments, it is likely that label incorporation represents the combination of viral and cellular protein accumulation and protein degradation resulting from autophagy. At both times, high levels of cellular proteins were observed in mock-infected cells (Fig. 4). As expected, viral proteins were abundant in 17termAR-infected MEFs at 6 h postinfection while synthesis of cellular proteins was low (Fig. 4). At 12 h postinfection with 17termAR, synthesis and accumulation of viral proteins were almost unchanged in Atg5−/− MEFs compared to 6 h following infection. In the Atg5+/+ cells, however, protein synthesis and accumulation were substantially lower at 12 h compared to 6 h postinfection with 17termAR. This increase in viral protein synthesis following 17termAR infection of Atg5−/− cells correlates with the slightly increased replication observed in these cells (Fig. 2A). In contrast, at both 6 and 12 h following infection with 17termA, significant amounts of cellular proteins accumulated, with viral proteins barely detectable (Fig. 4). Levels of cellular protein accumulation following infection with 17termA were slightly increased in Atg5−/− compared to Atg5+/+ cells. Increased protein accumulation in 17termA infected Atg5−/− cells was predominantly cellular, consistent with viral growth not being impacted by the absence of Atg5 (Fig. 2A). These studies suggest that accumulation of viral proteins in ICP34.5-null virus-infected cells results from translational arrest rather than autophagic degradation.
FIG. 4.
The lack of viral protein accumulation following infection with 17termA is due to translational arrest mediated by phosphorylated eIF2α rather than autophagy-dependent protein degradation. Shown is an autoradiograph of Atg5−/− and Atg5+/+ MEFs mock infected or infected with 17termAR or 17termA at a multiplicity of 5 PFU/cell. At 6 and 12 h postinfection, 35S-containing methionine and cysteine were incorporated into newly synthesized proteins for 2 h, harvested, and then visualized via autoradiography.
DISCUSSION
Upon invasion by a pathogen, the host may initiate autophagosome formation as a defense mechanism to sequester and eliminate pathogens and to promote cell survival. In infected primary MEFs and cultured murine superior cervical ganglion neurons, there are more virions within autophagosomes infected with ICP34.5-deficient virus than with wild-type virus (52). In PKR−/− cells, however, the number of autophagosome-engulfed virions lacking ICP34.5 is equivalent to the level of wild-type virus (52). Additionally, infection with HSV-1 lacking the 20-amino-acid Beclin 1 binding domain of ICP34.5 led to increased autophagosome formation following infection of superior cervical ganglion neurons compared to a marker-rescued virus (43). These studies suggest that ICP34.5 regulates autophagy through its interaction with the PKR/eIF2α pathway, as well as its through physical interaction with Beclin 1. Studies are currently under way to determine if the functions of Beclin 1 and PP1α binding by ICP34.5 are separable or integrated into a single pathway.
We have demonstrated that despite increased autophagosome formation following infection with an ICP34.5-null virus, elimination of the autophagic pathway does not alter its growth. Additionally, autophagy does not impact the in vitro replication of other mutant or control viruses. Additionally, Δ68H replicates similarly to its marker-rescued virus and is able to mediate eIF2α phosphorylation, further suggesting that replication defects of an ICP34.5-deficient virus in culture are due to high levels of phosphorylated eIF2α and subsequent shutoff of viral protein synthesis. The dephosphorylation of eIF2α and prevention of translational arrest is therefore the critical ICP34.5-dependent function for efficient HSV-1 replication. Although it has been suggested that autophagy decreases HSV-1 replication through xenophagic degradation of HSV-1, the impact of xenophagy may only be detectable under particular infection conditions or during infection in vivo (43).
Since eIF2α phosphorylation is required for starvation and rapamycin-induced conversion of LC3, we compared the phosphorylation states of eIF2α following the infection of autophagy-deficient and competent cells. An intact autophagic pathway did not affect the ability of ICP34.5 to mediate dephosphorylation of eIF2α, consistent with previous studies which indicate that eIF2α phosphorylation is upstream of autophagy induction (22). The phosphorylation state of eIF2α directly correlated with autophagosome formation in HSV-1-infected MEFs. Autophagy also had little impact upon the production of viral proteins following infection with both 17termAR and 17termA. Also, deletion of the 20-amino-acid Beclin 1 binding domain did not affect the ability of ICP34.5 to mediate dephosphorylation of eIF2α, and translation proceeded in the presence of viral infection (43). Thus, the lack of viral proteins observed following 17termA infection is mostly due to phosphorylated eIF2α and ensuing translational arrest rather than autophagy-induced protein degradation. These data provide evidence to support our conclusion that the key function of ICP34.5 to counteract PKR-mediated translational arrest.
Increased levels of phosphorylation of eIF2α correspond to increased autophagy in virus-infected cells. However, mock-infected cells have elevated levels of phosphorylated eIF2α compared to 17termAR-infected cells but fewer autophagosomes. It is possible that low levels of phosphorylated eIF2α may be sufficient to induce autophagy despite the presence of ICP34.5. Another explanation is that viral or virus-induced factors in addition to phosphorylated eIF2α must be present in order for autophagy to be induced. One such virus-induced factor may be IFN-γ, which has been shown to induce autophagy (10, 15). IFN-γ protein expression was induced immediately following corneal infection with HSV-1 (14). These in vivo observations may be relevant in vitro in that IFN-γ may be induced following HSV-1 infection of MEFs, leading to an increase in autophagosome formation.
An increase in autophagosome formation was observed following infection with an HSV-1 strain that lacked the Beclin 1 binding domain of ICP34.5 (43). ICP34.5-mediated regulation of autophagosome formation appears to be a separable function from that which mediates eIF2α dephosphorylation. While counteraction of translational shutoff via regulation of the PKR pathway is required for replication in vitro, autophagy antagonism may be advantageous under different cellular conditions. Alternatively, autophagy may be a more potent antiviral pathway in certain cell types and may provide the distinct advantage of being a relatively selective and nondestructive way to clear intracellular pathogens. In support of this hypothesis, the Beclin 1 binding domain mutant was neuroattenuated and unable to efficiently replicate in the brains of mice.
The ability of ICP34.5 to mediate the dephosphorylation of eIF2α and allow protein synthesis to proceed in the presence of activated PKR enables HSV-1 to replicate efficiently in MEFs. ICP34.5 can also decrease the numbers of autophagosomes and numbers of virions within autophagosomes. The contribution of these functions to viral replication and pathogenesis may depend upon the cellular environment in which the virus is present. Based upon the results of this and previous works, the interaction of viral proteins with the autophagic machinery involves multiple pathways which have only just begun to be characterized. Studies are currently under way to further clarify the roles of ICP34.5 and autophagy regulation on the pathogenesis of HSV-1.
Acknowledgments
We thank Darcy Gill of the Molecular Microbiology Imaging Facility for quantitation of autophagosomes and electron microscopy.
This work was supported by NIH grants to David A. Leib (RO1 EY09083) and Beth Levine (RO1 AI051367), the Department of Ophthalmology and Visual Sciences (P30 EY02687), and a National Institutes of Health, Institutional National Research Service Award (5-T32-EY13360-06) from the National Eye Institute and Research to Prevent Blindness. Support from Research to Prevent Blindness to the department and a Lew Wasserman Scholarship to David A. Leib is gratefully acknowledged.
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Ackermann, M., J. Chou, M. Sarmiento, R. A. Lerner, and B. Roizman. 1986. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J. Virol. 58:843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade, R. M., M. Wessendarp, M. J. Gubbels, B. Striepen, and C. S. Subauste. 2006. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J. Clin. Investig. 116:2366-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balliet, J. W., and P. A. Schaffer. 2006. Point mutations in herpes simplex virus type 1 oriL, but not in oriS, reduce pathogenesis during acute infection of mice and impair reactivation from latency. J. Virol. 80:440-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birmingham, C. L., A. C. Smith, M. A. Bakowski, T. Yoshimori, and J. H. Brumell. 2006. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281:11374-11383. [DOI] [PubMed] [Google Scholar]
- 5.Bolovan, C. A., N. M. Sawtell, and R. L. Thompson. 1994. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J. Virol. 68:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, S. M., J. Harland, A. R. MacLean, J. Podlech, and J. B. Clements. 1994. Cell type and cell state determine differential in vitro growth of non-neurovirulent ICP34.5-negative herpes simplex virus types 1 and 2. J. Gen. Virol. 75(Pt. 9):2367-2377. [DOI] [PubMed] [Google Scholar]
- 7.Brown, S. M., A. R. MacLean, J. D. Aitken, and J. Harland. 1994. ICP34.5 influences herpes simplex virus type 1 maturation and egress from infected cells in vitro. J. Gen. Virol. 75(Pt. 12):3679-3686. [DOI] [PubMed] [Google Scholar]
- 8.Chou, J., J.-J. Chen, M. Gross, and B. Roizman. 1995. Association of a Mr 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2α and premature shutoff of protein synthesis after infection with γ134.5− mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo, M. I. 2007. Autophagy: a pathogen driven process. IUBMB Life 59:238-242. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez, M. G., S. S. Master, S. B. Singh, G. A. Taylor, M. I. Colombo, and V. Deretic. 2004. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119:753-766. [DOI] [PubMed] [Google Scholar]
- 11.Harding, H. P., I. I. Novoa, Y. Zhang, H. Zeng, R. Wek, M. Schapira, and D. Ron. 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6:1099-1108. [DOI] [PubMed] [Google Scholar]
- 12.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, B. 2006. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 13:393-403. [DOI] [PubMed] [Google Scholar]
- 14.He, J., H. Ichimura, T. Iida, M. Minami, K. Kobayashi, M. Kita, C. Sotozono, Y. I. Tagawa, Y. Iwakura, and J. Imanishi. 1999. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV-1 infection. J. Interferon Cytokine Res. 19:609-615. [DOI] [PubMed] [Google Scholar]
- 15.Inbal, B., S. Bialik, I. Sabanay, G. Shani, and A. Kimchi. 2002. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J. Cell Biol. 157:455-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isaacs, A. and J. Lindenmann. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:258-267. [PubMed] [Google Scholar]
- 17.Jackson, W. T., T. H. Giddings, Jr., M. P. Taylor, S. Mulinyawe, M. Rabinovitch, R. R. Kopito, and K. Kirkegaard. 2005. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 3:e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing, X., M. Cerveny, K. Yang, and B. He. 2004. Replication of herpes simplex virus 1 depends on the γ134.5 functions that facilitate virus response to interferon and egress in the different stages of productive infection. J. Virol. 78:7653-7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jounai, N., F. Takeshita, K. Kobiyama, A. Sawano, A. Miyawaki, K. Q. Xin, K. J. Ishii, T. Kawai, S. Akira, K. Suzuki, and K. Okuda. 2007. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc. Natl. Acad. Sci. USA 104:14050-14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabeya, Y., N. Mizushima, T. Ueno, A. Yamamoto, T. Kirisako, T. Noda, E. Kominami, Y. Ohsumi, and T. Yoshimori. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19:5720-5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kihara, A., Y. Kabeya, Y. Ohsumi, and T. Yoshimori. 2001. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2:330-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kouroku, Y., E. Fujita, I. Tanida, T. Ueno, A. Isoai, H. Kumagai, S. Ogawa, R. J. Kaufman, E. Kominami, and T. Momoi. 2007. ER stress (PERK//eIF2α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 14:230-239. [DOI] [PubMed] [Google Scholar]
- 23.Kuma, A., M. Hatano, M. Matsui, A. Yamamoto, H. Nakaya, T. Yoshimori, Y. Ohsumi, T. Tokuhisa, and N. Mizushima. 2004. The role of autophagy during the early neonatal starvation period. Nature 432:1032. [DOI] [PubMed] [Google Scholar]
- 24.Lebleu, B., G. C. Sen, S. Shaila, B. Cabrer, and P. Lengyel. 1976. Interferon, double-stranded RNA, and protein phosphorylation. Proc. Natl. Acad. Sci. USA 73:3107-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang, X. H., L. K. Kleeman, H. H. Jiang, G. Gordon, J. E. Goldman, G. Berry, B. Herman, and B. Levine. 1998. Protection against fatal Sindbis virus encephalitis by Beclin, a novel Bcl-2-interacting protein. J. Virol. 72:8586-8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling, Y. M., M. H. Shaw, C. Ayala, I. Coppens, G. A. Taylor, D. J. Ferguson, and G. S. Yap. 2006. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J. Exp. Med. 203:2063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, Y., M. Schiff, K. Czymmek, Z. Talloczy, B. Levine, and S. P. Dinesh-Kumar. 2005. Autophagy regulates programmed cell death during the plant innate immune response. Cell 121:567-577. [DOI] [PubMed] [Google Scholar]
- 30.Luzikov, V. N. 1999. Quality control: from molecules to organelles. FEBS Lett. 448:201-205. [DOI] [PubMed] [Google Scholar]
- 31.Mao, H., and K. S. Rosenthal. 2003. Strain-dependent structural variants of herpes simplex virus type 1 ICP34.5 determine viral plaque size, efficiency of glycoprotein processing, and viral release and neuroinvasive disease potential. J. Virol. 77:3409-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meurs, E., K. Chong, J. Galabru, N. S. Thomas, I. M. Kerr, B. R. Williams, and A. G. Hovanessian. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62:379-390. [DOI] [PubMed] [Google Scholar]
- 33.Mizushima, N., H. Sugita, T. Yoshimori, and Y. Ohsumi. 1998. A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J. Biol. Chem. 273:33889-33892. [DOI] [PubMed] [Google Scholar]
- 34.Mizushima, N., A. Yamamoto, M. Hatano, Y. Kobayashi, Y. Kabeya, K. Suzuki, T. Tokuhisa, Y. Ohsumi, and T. Yoshimori. 2001. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 152:657-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushima, N., T. Yoshimori, and Y. Ohsumi. 2003. Role of the Apg12 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 35:553-561. [DOI] [PubMed] [Google Scholar]
- 36.Mizushima, N., T. Noda, T. Yoshimori, Y. Tanaka, T. Ishii, M. D. George, D. J. Klionsky, M. Ohsumi, and Y. Ohsumi. 1998., A protein conjugation system essential for autophagy. Nature 395:395-398. [DOI] [PubMed] [Google Scholar]
- 37.Mortimore, G. E., and A. R. Poso. 1987. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu. Rev. Nutr. 7:539-564. [DOI] [PubMed] [Google Scholar]
- 38.Mulvey, M., J. Poppers, D. Sternberg, and I. Mohr. 2003. Regulation of eIF2α phosphorylation by different functions that act during discrete phases in the herpes simplex virus type 1 life cycle. J. Virol. 77:10917-10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa, I., A. Amano, N. Mizushima, A. Yamamoto, H. Yamaguchi, T. Kamimoto, A. Nara, J. Funao, M. Nakata, K. Tsuda, S. Hamada, and T. Yoshimori. 2004. Autophagy defends cells against invading group A streptococcus. Science 306:1037-1040. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima, A., N. Tanaka, K. Tamai, M. Kyuuma, Y. Ishikawa, H. Sato, T. Yoshimori, S. Saito, and K. Sugamura. 2006. Survival of parvovirus B19-infected cells by cellular autophagy. Virology 349:254-263. [DOI] [PubMed] [Google Scholar]
- 41.Natarajan, K., M. R. Meyer, B. M. Jackson, D. Slade, C. Roberts, A. G. Hinnebusch, and M. J. Marton. 2001. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell. Biol. 21:4347-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogawa, M., T. Yoshimori, T. Suzuki, H. Sagara, N. Mizushima, and C. Sasakawa. 2005. Escape of intracellular Shigella from autophagy. Science 307:727-731. [DOI] [PubMed] [Google Scholar]
- 43.Orvedahl, A., D. Alexander, Z. Talloczy, Q. Sun, Y. Wei, W. Zhang, D. Burns, D. A. Leib, and B. Levine. 2007. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:37-49. [DOI] [PubMed] [Google Scholar]
- 44.Pasieka, T. J., T. Baas, V. S. Carter, S. C. Proll, M. G. Katze, and D. A. Leib. 2006. Functional genomic analysis of herpes simplex virus type 1 counteraction of the host innate response. J. Virol. 80:7600-7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perng, G. C., H. Ghiasi, S. M. Slanina, A. B. Nesburn, and S. L. Wechsler. 1996. High-dose ocular infection with a herpes simplex virus type 1 ICP34.5 deletion mutant produces no corneal disease or neurovirulence yet results in wild-type levels of spontaneous reactivation. J. Virol. 70:2883-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prentice, E., W. G. Jerome, T. Yoshimori, N. Mizushima, and M. R. Denison. 2004. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 279:10136-10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rader, K. A., C. E. Ackland-Berglund, J. K. Miller, J. S. Pepose, and D. A. Leib. 1993. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J. Gen. Virol. 74(Pt. 9):1859-1869. [DOI] [PubMed] [Google Scholar]
- 48.Ramaiah, K. V., M. V. Davies, J.-J. Chen, and R. J. Kaufman. 1994. Expression of mutant eukaryotic initiation factor 2 α subunit (eIF-2α) reduces inhibition of guanine nucleotide exchange activity of eIF-2B mediated by eIF-2α phosphorylation. Mol. Cell. Biol. 14:4546-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts, W. K., A. Hovanessian, R. E. Brown, M. J. Clemens, and I. M. Kerr. 1976. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature 264:477-480. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki, K., T. Kirisako, Y. Kamada, N. Mizushima, T. Noda, and Y. Ohsumi. 2001. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 20:5971-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talloczy, Z., W. Jiang, H. I. Virgin, D. A. Leib, D. Scheuner, R. J. Kaufman, E. L. Eskelinen, and B. Levine. 2002. Regulation of starvation- and virus-induced autophagy by the eIF2α kinase signaling pathway. Proc. Natl. Acad. Sci. USA 99:190-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tallóczy, Z., H. W. I. Virgin, and B. Levine. 2006. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy 2:24-29. [DOI] [PubMed] [Google Scholar]
- 53.Reference deleted.
- 54.Tanida, I., T. Ueno, and E. Kominami. 2004. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 36:2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trgovcich, J., D. Johnson, and B. Roizman. 2002. Cell surface major histocompatibility complex class II proteins are regulated by the products of the γ134.5 and UL41 genes of herpes simplex virus 1. J. Virol. 76:6974-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward, S. L., D. Scheuner, J. Poppers, R. J. Kaufman, I. Mohr, and D. A. Leib. 2003. In vivo replication of an ICP34.5 second-site suppressor mutant following corneal infection correlates with in vitro regulation of eIF2α phosphorylation. J. Virol. 77:4626-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wek, R. C., H. Y. Jiang, and T. G. Anthony. 2006. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 34(Pt. 1):7-11. [DOI] [PubMed] [Google Scholar]
- 58.Weller, S. K., A. Spadaro, J. E. Schaffer, A. W. Murray, A. M. Maxam, and P. A. Schaffer. 1985. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol. Cell. Biol. 5:930-942. [DOI] [PMC free article] [PubMed] [Google Scholar]