Abstract
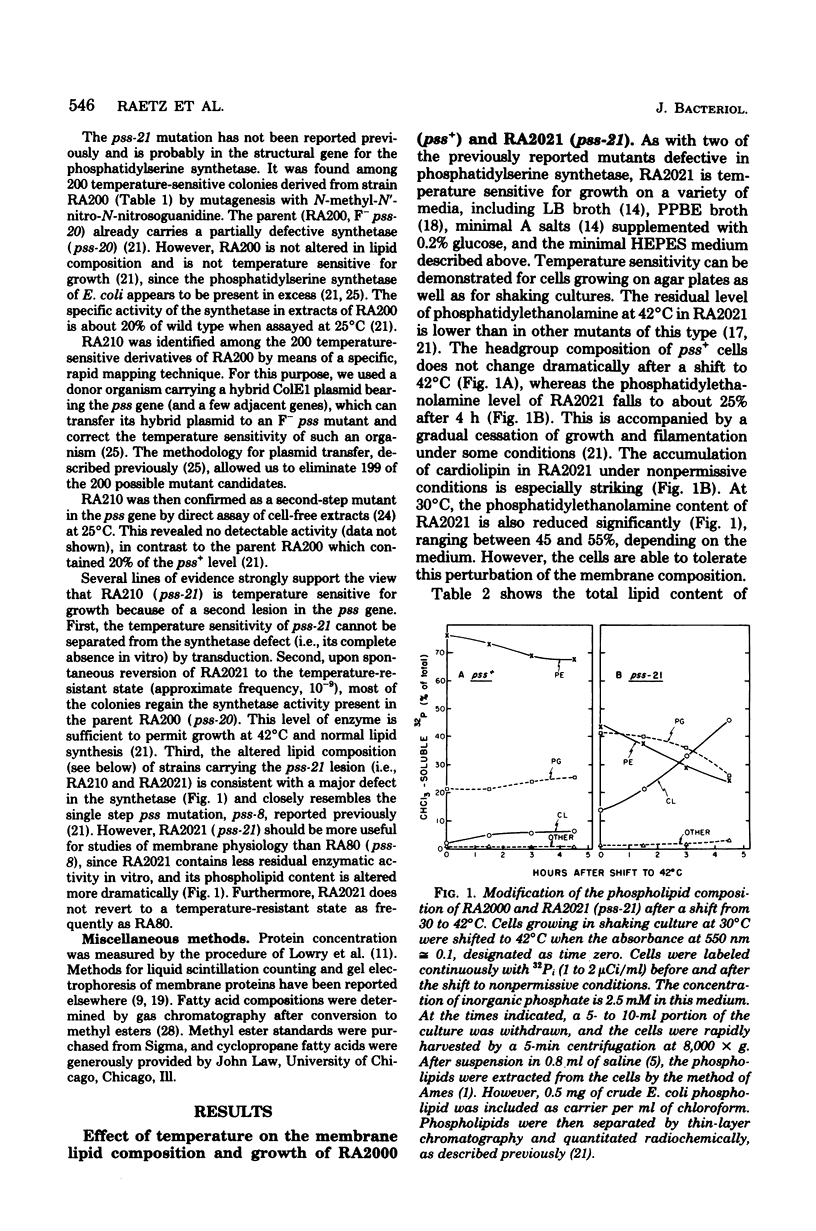
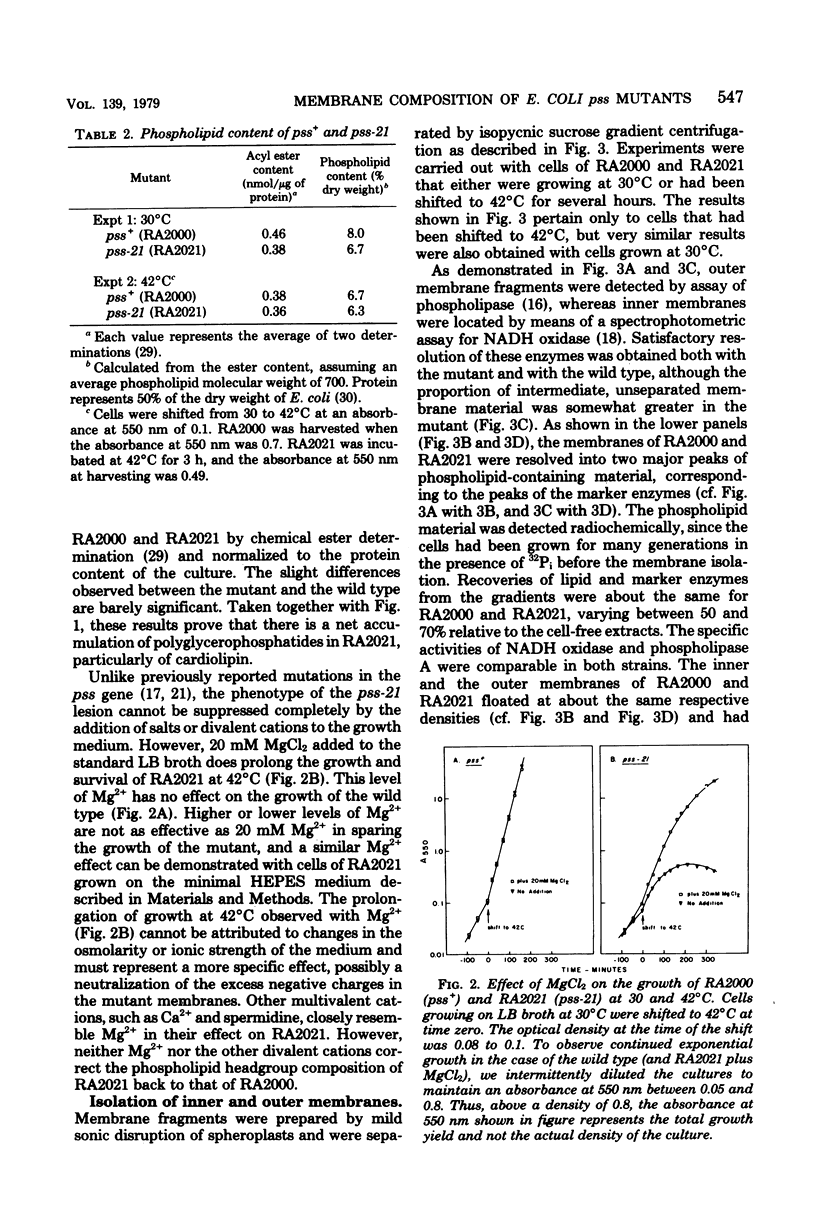
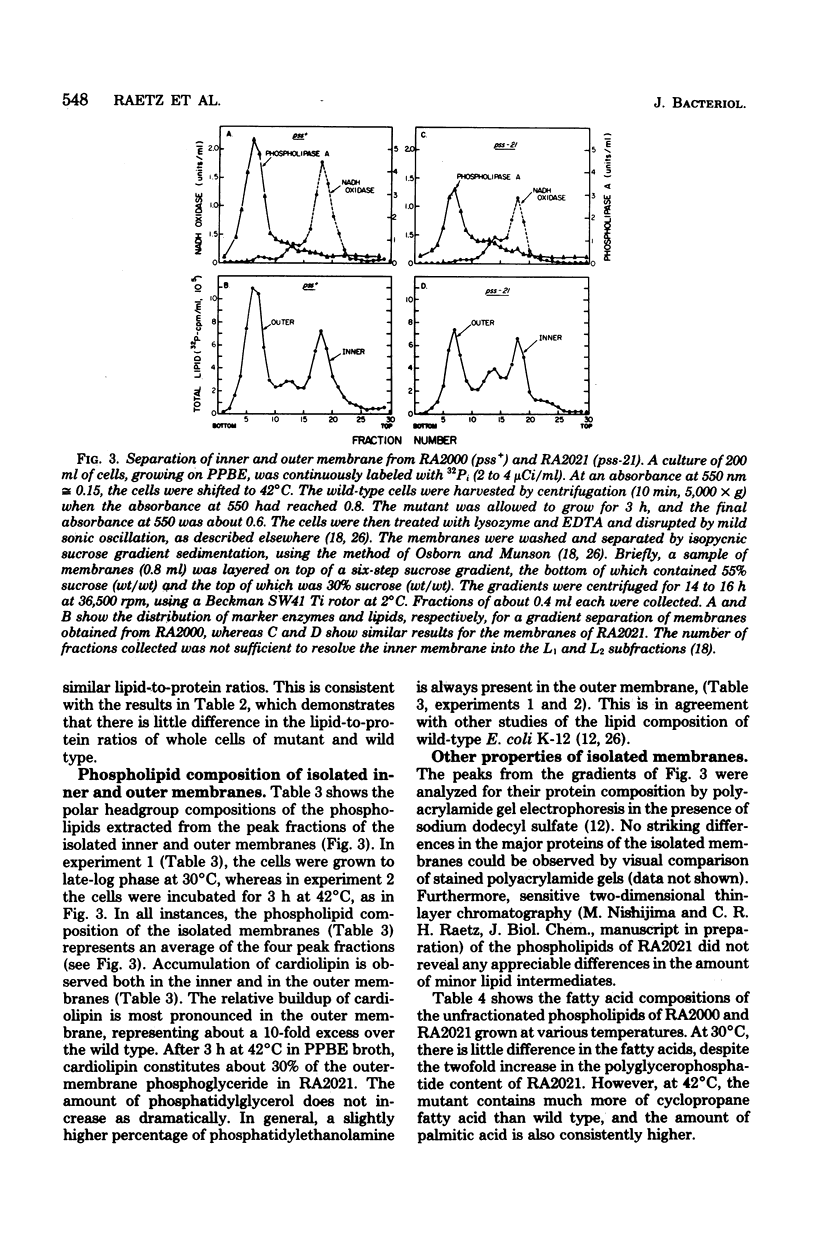
Mutants of Escherichia coli defective in phosphatidylserine synthetase (pss) make less phosphatidylethanolamine than normal cells, and they are temperature sensitive for growth. We have isolated a new mutant, designated RA2021, which is better than previously available strains in that the residual phosphatidylethanolamine level approaches 25% after 4 h at 42°C. The total amount of phospholipid normalized to the density of the culture is about the same in RA2021 (pss-21) as in the isogenic wild-type RA2000 (pss+). Consequently, there is a net accumulation of polyglycerophosphatides in the mutant, particularly of cardiolipin. The addition of 10 to 20 mM MgCl2 to a culture of RA2021 prolongs growth under nonpermissive conditions and prevents loss of cell viability, but it does not eliminate the temperature-sensitive phenotype. Divalent cations, like Mg2+, do not correct the phospholipid composition of the mutant, but may act indirectly by balancing the negative charges of phosphatidylglycerol and cardiolipin. To determine the effects of the pss mutation on membrane composition, we have examined the subcellular distribution of the polyglycerophosphatides that accumulate in these strains. All of the excess anionic lipids of RA2021 are associated with the envelope fraction and are distributed equally between the inner and outer membranes. The protein compositions of the isolated membranes do not differ significantly in the mutant and wild type. The fatty acid composition of RA2021 is almost the same as wild type at 30°C, but there is more palmitic and cyclopropane fatty acid at 42°C. These results demonstrate that the modification of the polar lipid composition observed in pss mutants affects both membranes and that cardiolipin, which is not ordinarily present in large quantities, can accumulate in the outer membrane when it is overproduced by the cell. The altered polar headgroup composition of the outer membrane in pss mutants may account, in part, for their hypersensitivity to the aminoglycoside antibiotics.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M., Mavis R. D., Osborn M. J., Vagelos P. R. Enzymes of phospholipid metabolism: localization in the cytoplasmic and outer membrane of the cell envelope of Escherichia coli and Salmonella typhimurium. Biochim Biophys Acta. 1971 Dec 3;249(2):628–635. doi: 10.1016/0005-2736(71)90144-1. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Biogenesis of membrane lipids: mutants of Escherichia coli with temperature-sensitive phosphatidylserine decarboxylase. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1112–1116. doi: 10.1073/pnas.72.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Interaction of Salmonella typhimurium with phospholipid vesicles. Incorporation of exogenous lipids into intact cells. J Biol Chem. 1977 Oct 25;252(20):7398–7404. [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J., Peters R. Distribution of lipids in cytoplasmic and outer membranes of Escherichia coli K12. Biochim Biophys Acta. 1976 Jul 20;441(1):38–47. doi: 10.1016/0005-2760(76)90279-4. [DOI] [PubMed] [Google Scholar]
- McIntyre T. M., Bell R. M. Escherichia coli mutants defective in membrane phospholipid synthesis: binding and metabolism of 1-oleoylglycerol 3-phosphate by a plsB deep rough mutant. J Bacteriol. 1978 Jul;135(1):215–226. doi: 10.1128/jb.135.1.215-226.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nishijima M., Nakaike S., Tamori Y., Nojima S. Detergent-resistant phospholipase A of Escherichia coli K-12. Purification and properties. Eur J Biochem. 1977 Feb 15;73(1):115–124. doi: 10.1111/j.1432-1033.1977.tb11297.x. [DOI] [PubMed] [Google Scholar]
- Ohta A., Shibuya I. Membrane phospholipid synthesis and phenotypic correlation of an Escherichia coli pss mutant. J Bacteriol. 1977 Nov;132(2):434–443. doi: 10.1128/jb.132.2.434-443.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Pluschke G., Hirota Y., Overath P. Function of phospholipids in Escherichia coli. Characterization of a mutant deficient in cardiolipin synthesis. J Biol Chem. 1978 Jul 25;253(14):5048–5055. [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Foulds J. Envelope composition and antibiotic hypersensitivity of Escherichia coli mutants defective in phosphatidylserine synthetase. J Biol Chem. 1977 Aug 25;252(16):5911–5915. [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. The association of phosphatidylserine synthetase with ribosomes in extracts of Escherichia coli. J Biol Chem. 1972 Apr 10;247(7):2008–2014. [PubMed] [Google Scholar]
- Raetz C. R., Larson T. J., Dowhan W. Gene cloning for the isolation of enzymes of membrane lipid synthesis: phosphatidylserine synthase overproduction in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1412–1416. doi: 10.1073/pnas.74.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Newman K. F. Diglyceride kinase mutants of Escherichia coli: inner membrane association of 1,2-diglyceride and its relation to synthesis of membrane-derived oligosaccharides. J Bacteriol. 1979 Feb;137(2):860–868. doi: 10.1128/jb.137.2.860-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Phosphatidylserine synthetase mutants of Escherichia coli. Genetic mapping and membrane phospholipid composition. J Biol Chem. 1976 Jun 10;251(11):3242–3249. [PubMed] [Google Scholar]
- STERN I., SHAPIRO B. A rapid and simple method for the determination of esterified fatty acids and for total fatty acids in blood. J Clin Pathol. 1953 May;6(2):158–160. doi: 10.1136/jcp.6.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert D. F. Genetic modification of membrane lipid. Annu Rev Biochem. 1975;44:315–339. doi: 10.1146/annurev.bi.44.070175.001531. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Ruch F., Vagelos P. R. Fatty acid replacements in a fatty acid auxotroph of Escherichia coli. J Bacteriol. 1968 May;95(5):1658–1665. doi: 10.1128/jb.95.5.1658-1665.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. A., Albright F. R., Lennarz W. J., Schnaitman C. A. Distribution of phospholipid-synthesizing enzymes in the wall and membrane subfractions of the envelope of Escherichia coli. Biochim Biophys Acta. 1971 Dec 3;249(2):636–642. doi: 10.1016/0005-2736(71)90145-3. [DOI] [PubMed] [Google Scholar]


