Abstract
While recombinant adeno-associated virus (rAAV) vectors promote long-term transgene expression in the lungs and other organs, the goal of correcting chronic inherited lung diseases such as cystic fibrosis with this type of viral gene transfer vector is limited by the requirement of achieving stable potent transgene expression, potentially requiring vector readministration. Here we evaluated the abilities of rAAV type 5/5 (rAAV5/5) vectors based on the genome and capsid of AAV5 to efficiently transduce the lungs and nasal epithelium of mice after repeated administration. Transduction efficiency as judged by reporter gene expression was markedly reduced on a second rAAV5/5 administration and effectively abolished on a third. Varying the period between administrations from 8 to 36 weeks did not allow efficient repeated administration. A rapid rise in anti-AAV5 antibodies was noted after rAAV5/5 vector administration that was sustained for the entire period of investigation (in some cases exceeding 9 months). Furthermore, this antibody response and subsequent failure to repeatedly administer the vector were not rescued by the in vivo expression of CTLA4Ig from an rAAV5/5 vector. These results suggest that without the development of an effective and clinically acceptable immunosuppression strategy, treatments for chronic diseases that require repeated administration of rAAV5/5 vectors will be unsuccessful.
Recombinant gene transfer vectors based on recombinant adeno-associated virus (rAAV) direct efficient transgene expression in a wide range of tissues, including muscle (31), lungs (15), liver (20), brain (9), and retina (2). The popularity of rAAV vectors lies in part with the observations that wild-type AAV is not pathogenic to humans and is relatively nonimmunogenic (7). As a result, several clinical trials involving rAAV2 vectors have been initiated, most notably involving patients with cystic fibrosis (CF) (1, 11, 29) and hemophilia (19).
The lungs are an attractive organ for gene delivery, since they are readily accessible by minimally invasive procedures, such as bronchoscopy and nebulization. We are interested in using rAAV vectors to treat chronic inherited lung diseases such as CF. Here we have focused on the use of rAAV type 5/5 (rAAV5/5) vectors (6), which transduce lung cells more efficiently than vectors based on the more widely used rAAV2/2 (26, 27, 32). Crucially, to treat chronic inherited disease, it is likely to be necessary to maintain transgene expression throughout the life of the treated individual. To date there are no definitive data that measure the turnover of different cell types in the lungs of either mice or humans. We do know, however, that the majority of cells in the lungs are terminally differentiated and slowly replaced; therefore, it is expected that transgene expression from the single administration of any gene transfer vector will fall with time unless a cell population with the ability to self-renew is targeted with a vector capable of replication or integration. Regrettably, lung stem cell(s) remain poorly characterized, and the “progenitor-progeny” relationships that are well understood from continuously proliferating tissues such as bone marrow may well not apply (24). We have observed that rAAV5/5 directs murine lung transgene expression that peaks approximately 1 month after administration and falls to only ∼50% of this peak after 12 months (27). This stands as an unexplained paradox relative to one published report where the turnover time of cells in the conducting airway epithelia was found to be approximately 3 months (4). Nevertheless, the gradual decline in transgene expression seen with our mouse lung model suggests that it will be important to efficiently readminister the vector in order to achieve lifelong transgene expression.
There is contradictory evidence regarding the efficiency with which viral vectors can be repeatedly administered. Multiple studies with a variety of organs have highlighted the difficulties of efficient second or third administrations of rAAV2/2 vectors (15, 31). Reduced efficacy on repeated administration has largely correlated with the generation of a neutralizing immune response that inhibits successive rounds of transduction (16). A number of studies have reported successful repeated administration after pretreatment of the host with immunosuppressing antibodies, including anti-CD4 and/or anti-CD40L (23), and/or immune-modulating agents, including CTLA4Ig (16), or broad immunosuppressing agents, such as cyclophosphamide (5). In a refinement of this strategy, repeated administration of a recombinant adenoviral vector has been achieved by the construction of a virus that simultaneously expresses both the transgene of interest and CTLA4Ig (28). In contrast, two studies have observed that rAAV may be efficiently administered a second time if the time between administrations is extended beyond approximately 28 weeks and the capsid is derived from AAV5 or AAV9 (3, 21).
In the present study, we thoroughly evaluated the efficacy of rAAV5/5 after repeated administration to the nose and lungs of BALB/c mice. We showed that rAAV5/5 administration provoked the production of sustained anti-AAV5 capsid-neutralizing antibodies that substantially lowered lung gene transfer on a second administration and effectively abolished it on a third. Importantly, neither the local expression of CTLA4Ig nor an increase in the period between vector administrations from 8 to 36 weeks improved the efficacy of second or third administrations of rAAV5/5.
MATERIALS AND METHODS
Cells and plasmids.
Human embryonic kidney (HEK) 293T cells (13) and African green monkey kidney COS7 cells (European Collection of Cell Cultures) were maintained as described previously (27).
The rAAV5 vector plasmid pAAV5/5Lux, containing the ITRs of AAV5 separated by the firefly luciferase coding region from pGL3 (Lux) (Promega, Southampton, United Kingdom) under the transcriptional control of the cytomegalovirus immediate-early enhancer/promoter, has been described previously (27). Other vector plasmids were derived from pAAV5/5Lux by replacing the Lux coding region with the coding region of enhanced green fluorescent protein (EGFP) from pEGFP-N1 (Clontech, BD Bioscience, Cowley, United Kingdom), the chloramphenicol acetyltransferase (CAT) coding region from pREP4CAT (Invitrogen, Paisley, United Kingdom), and the chimeric cytotoxic T-lymphocyte-associated antigen 4-human IgG Fc (CTLA4Ig) coding region from pCDM8-CTLA4Ig (American Type Culture Collection clone 68629). Plasmid stocks of p5RC (AAV5 helper plasmid) (6) and all vector plasmids were prepared using QIAGEN Endofree Megaprep kits (QIAGEN, Crawley, United Kingdom). pAd12 (adenovirus helper plasmid) (6) was manufactured by Bayou Labs (Harahan, LA).
Virus production and characterization.
Recombinant AAV5 was produced by transient triple plasmid transfection of HEK 293T cells and purified by CsCl density gradient ultracentrifugation followed by dialysis against D-phosphate-buffered saline (PBS) (27). Virus was stored at −80°C in 100- to 200-μl aliquots. Recombinant AAV5 titers (expressed in DNase-resistant genome copies [GC]/ml) were determined by TaqMan real-time PCR analysis of virus genomes (27). The rAAV2/2GFP vector was supplied by the Vector Core at the Gene Therapy Program (University of Pennsylvania). Function of the expression cassettes in virus preparations was confirmed by determination of reporter gene activity or by enzyme-linked immunosorbent assay (ELISA) following transduction of COS7 cells with 1 × 1010 GC of virus.
Animal studies.
Female BALB/c mice of ages 6 to 8 weeks were used throughout. Mice were housed in accordance with United Kingdom Home Office ethical and welfare guidelines and fed standard chow and water ad libitum. Nasal perfusion, lung delivery (nasal insufflation), tissue harvesting, and determination of protein levels and luciferase activities in nasal and lung samples were done as described previously (12, 27). Note that the background luciferase activity in naive nasal samples is typically ∼10-fold higher than that in naive lung samples due to differences in protein content. For comparative purposes, under the luciferase assay conditions used, 10 relative light units (RLU) equates to approximately 260 ng of recombinant luciferase (Promega).
Detection of anti-AAV5, anti-Lux, anti-CAT, anti-green fluorescent protein (GFP), and anti-CTLA4Ig antibodies in pooled sera by ELISA.
Levels of anti-AAV5, anti-Lux, anti-CAT, anti-GFP, and anti-CTLA4Ig antibodies in pooled sera of treated mice were quantified using Strip-well plates (Costar; VWR International Ltd., Lutterworth, United Kingdom) coated with 1 × 109 GC rAAV5/5Lux, 0.5 μg recombinant Lux (Promega), 0.25 μg CAT (Sigma, Poole, United Kingdom), 1 μg heat-denatured (90°C 5′) recombinant GFP (Clontech-Takara Bio Europe, Saint-Germain-en-Laye, France), or 0.5 μg rhCTLA4Ig (R&D Systems, Abingdon, United Kingdom) per well as described previously (27). Where the calculated antibody titer was outside the range of serum dilutions tested, i.e., below 20 or above 20,480, it is presented as being 20 or 20,480, respectively, on the graphs.
Statistical analyses.
Statistical power analysis was used to assist group size selection, to allow statistical significance with a twofold reduction in peak luciferase activity with >0.8 power. Luciferase activity data are expressed in relative light units per mg total protein as the mean ± standard error of the mean (SEM). Results were analyzed using analysis of variance with log-transformed data for luciferase activity and with untransformed data for Ig levels determined by ELISA. Statistical significance of Fisher's protected least significant difference post hoc tests was accepted with P values of <0.05.
RESULTS
rAAV5/5 cannot be efficiently repeatedly administered to the mouse lung with an 8-week dosing interval.
To investigate the feasibility of repeatedly administering rAAV5/5 to the lung, groups of mice were exposed to one, two, or three doses of 1 × 1011 DNase-resistant GC of virus at 8-week intervals. Throughout these studies, we utilized rAAV5/5 viruses expressing alternate transgenes (rAAV5/5CAT, -EGFP, and -Lux) to ensure that any immune block on subsequent virus administration was not due to the expressed gene products. In each case, the final viral administration was of rAAV5/5Lux, with lung luciferase activity being measured 4 weeks after delivery. Four treatment regimes were compared: a single administration of rAAV5/5Lux; two sham administrations of vehicle (PBS) followed by rAAV5/5Lux; a sham administration followed by rAAV5/5EGFP and subsequently rAAV5/5Lux, and administration of rAAV5/5CAT followed by rAAV5/5EGFP and subsequently rAAV5/5Lux. Animals exposed to three sham administrations served as negative controls (Fig. 1).
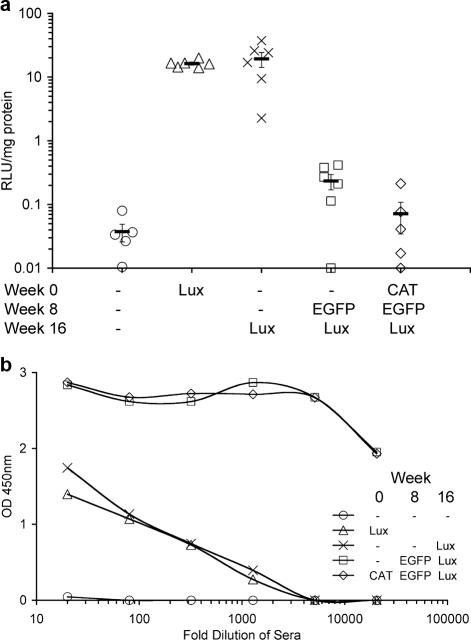
FIG. 1.
Repeat administration of rAAV5/5 to mouse lungs at 8-week intervals. Female BALB/c mice (n = 5 to 6 per group) were anesthetized, and 150 μl of virus solution containing 1 × 1011 GC rAAV5/5CAT, rAAV5/5EGFP, or rAAV5/5Lux was administered to the lungs at the times indicated. Sham administration of vehicle (PBS) is indicated by a dash. Lung tissue for determination of luciferase activity and blood for determination of anti-AAV5 antibodies were harvested at week 4 for the animals that received a single administration of rAAV5/5Lux and at week 20 for all other groups. (a) Lung tissue homogenates were assayed for luciferase activity, expressed as arbitrary relative light units per mg of lung protein. The symbols (consistent for the same treatment group between panels) indicate individual mice, and the bar indicates the group mean (± SEM). (b) Serum from the blood was isolated, and pooled sera for each group were serially diluted and assayed for anti-AAV5 mouse IgG, IgM, and IgA by ELISA. OD 450nm, optical density at 450 nm.
Consistent with our previously published studies, delivery of a single dose of rAAV5/5 to the mouse lung resulted in significant lung reporter gene expression (27). Similar levels of lung luciferase activity were noted in animals that received a single administration of rAAV5/5Lux and animals that received two sham administrations followed by rAAV5/5Lux (19.33 ± 5.12 and 16.31 ± 0.88 RLU/mg protein, respectively; P = 0.74) (Fig. 1a), indicating that repeated cycles of sham administration, including anesthesia and delivery of PBS to the lung, did not affect the outcome measure. Importantly, lung luciferase activity in the group of animals that received two doses of rAAV5/5 (sham administration followed by rAAV5/5EGFP and rAAV5/5Lux) was significantly reduced (0.23 ± 0.06 RLU/mg protein) compared to that of animals that received two sham administrations followed by rAAV5/5Lux (P < 0.0001) (Fig. 1a). Crucially, when mice were exposed to a total of three virus doses, lung luciferase activity was indistinguishable from that of mice receiving three sham administrations (0.07 ± 0.04 and 0.04 ± 0.01 RLU/mg protein, respectively; P = 0.91) (Fig. 1a).
Failure to transduce mouse lung with rAAV5/5 correlates with anti-AAV5 serum antibody levels.
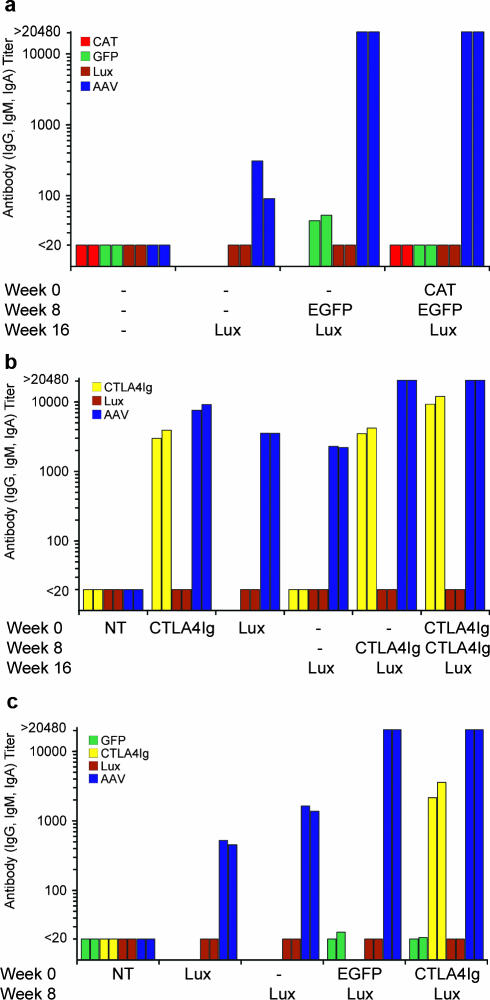
Sera from the mice in the study described in Fig. 1a were analyzed for anti-AAV antibodies. All groups of mice except those receiving sham treatment had detectable levels of antibodies against rAAV5/5Lux (Fig. 1b). Importantly, higher anti-AAV5 antibody titers were observed in the groups of animals exposed to more than one dose of virus (titers of >1:20,480 after two or three exposures and 1:90 to 1:328 for a single exposure) (Fig. 1b).
Increasing the dosing interval from 8 to 36 weeks does not improve transduction by a second dose of rAAV5/5.
Despite the slow decline in lung luciferase activity noted following delivery of a single dose of rAAV5/5Lux to the mouse lung (27), substantial transgene expression was still detectable up to 1 year postadministration. Consequently, the delivery of subsequent doses of virus to boost transgene expression need not necessarily be as early as the 8-week interval allowed in the study described in the legend to Fig. 1.
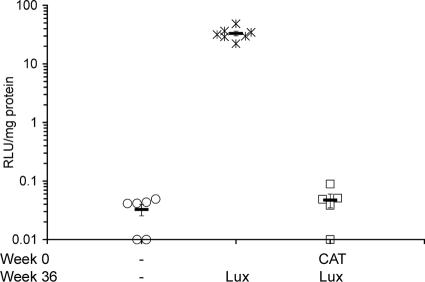
To investigate if a longer interval between dosing events might improve transduction efficiency, groups of mice were exposed to either sham administration or 1 × 1011 GC of rAAV5/5CAT followed by 1 × 1011 GC of rAAV5/5Lux 36 weeks later. Animals exposed to two sham administrations with a 36-week interval served as negative controls (Fig. 2). Despite the increase in the dosing interval from 8 to 36 weeks, a second dose of rAAV5/5 directed lung luciferase activity that was indistinguishable from that of sham-treated animals (0.05 ± 0.01 and 0.03 ± 0.01 RLU/mg protein, respectively; P = 0.866) (Fig. 2). Importantly, mice receiving sham administration followed by rAAV5/5Lux with a 36-week interval had robust lung luciferase activity (33.10 ± 3.07 RLU/mg protein) (Fig. 2). As in the study conducted with an 8-week dosing interval, high levels of anti-AAV5 serum antibodies were observed for the group of animals receiving two doses of rAAV5/5 (titer of >1:20,480) compared with those for naive mice (titer of <1:20).
FIG. 2.
Repeat administration of rAAV5/5 to mouse lungs at a 36-week interval. Female BALB/c mice (n = 5 to 7 per group) were anesthetized, and 150 μl of virus solution containing 1 × 1011 GC rAAV5/5CAT or rAAV5/5Lux was administered to the lungs at the times indicated. Sham administration is indicated by a dash. Lung tissue for determination of luciferase was harvested at week 40 for all groups. Lung tissue homogenates were assayed for luciferase activity expressed as arbitrary relative light units per mg of lung protein. The symbols indicate individual mice, and the bar indicates the group mean (± SEM).
Delivery of rAAV5/5 encoding CTLA4Ig does not prevent generation of anti-AAV5 serum antibodies and does not allow transduction by a second or third dose of rAAV5/5.
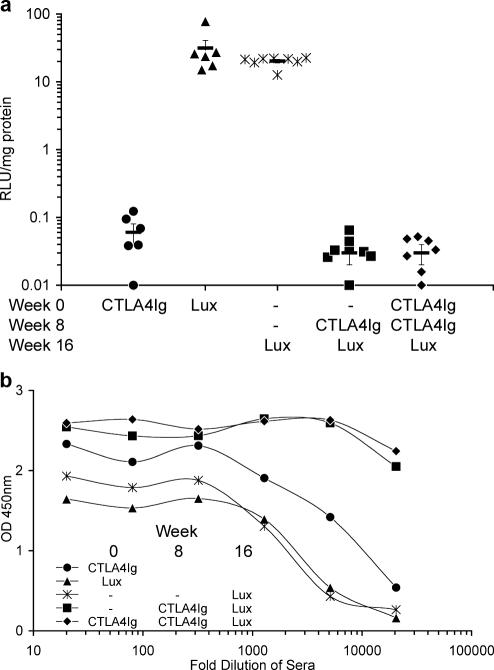
To investigate if repeated administration of rAAV5/5 could be supported by expression of a transgene with immunosuppressing properties, we examined whether rAAV5/5 encoding a soluble form of CTLA4Ig (rAAV5/5CTLA4Ig) might block the generation of antibodies against rAAV5/5 components. Five treatment regimes (1 × 1011 GC rAAV5/5 per dose, with an 8-week dosing interval) were compared: a single administration of rAAV5/5CTLA4Ig; a single administration of rAAV5/5Lux; two sham administrations followed by rAAV5/5Lux; sham administration followed by rAAV5/5CTLA4Ig and subsequently rAAV5/5Lux; and two administrations of rAAV5/5CTLA4Ig followed by rAAV5/5Lux (Fig. 3). Importantly, similar, robust levels of lung luciferase activity were noted for animals that received a single administration of rAAV5/5Lux and animals that received two sham administrations followed by rAAV5/5Lux (31 ± 9.61 and 20 ± 1.16 RLU/mg protein, respectively) (Fig. 3a). Crucially, neither group receiving rAAV5/5CTLA4Ig followed by rAAV5/5Lux had lung luciferase activity above that of animals receiving only rAAV5/5CTLA4Ig (both 0.03 ± 0.01 RLU/mg protein, compared with 0.06 ± 0.02 RLU/mg protein for rAAV5/5CTLA4Ig only; P = 0.81 for both) (Fig. 3a). This suggested that the local expression of CTLA4Ig did not prevent an immune response against rAAV5/5 particles. This was confirmed by an analysis of the sera from the same mice, showing that all experimental groups had high levels of antibodies against rAAV5/5, with the multiple exposure leading to the highest titers, only just starting to decrease at a dilution of 1:20,480 (Fig. 3b).
FIG. 3.
Repeat administration of rAAV5/5CTLA4Ig to mouse lungs. Female BALB/c mice (n = 6 to 8 per group) were anesthetized, and 150 μl of virus solution containing 1 × 1011 GC rAAV5/5CTLA4Ig or rAAV5/5Lux was administered to the lungs at the times indicated. Sham administration is indicated by a dash. Lung tissue for determination of luciferase activity and blood for determination of anti-AAV5 antibodies were harvested at week 4 for the animals that received a single administration of rAAV5/5CTLA4Ig or rAAV5/5Lux and at week 20 for all other groups. (a) Lung tissue homogenates were assayed for luciferase activity, expressed as arbitrary relative light units per mg of lung protein. The symbols (consistent for the same treatment group between panels) indicate individual mice, and the bar indicates the group mean (± SEM). (b) Serum from the blood was isolated, and pooled sera for each group were serially diluted and assayed for anti-AAV5 mouse IgG, IgM, and IgA by ELISA. OD 450nm, optical density at 450 nm.
Delivery of rAAV5/5 to the nasal epithelium does not allow efficient repeated transduction and does not circumvent the generation of anti-AAV5 antibodies.
In the preceding studies, rAAV5/5 was administered to the lung as a 150-μl bolus that by itself may have led to a host inflammatory response that may have augmented the specific immune responses observed. To address this, we investigated repeat delivery of rAAV5/5 to respiratory tissue with an alternative delivery system where the virus is applied to the nasal epithelium by slow perfusion (27). For simplicity, this study was restricted to a maximum of two viral administrations. Four treatment regimes (1 × 1011 GC rAAV5/5 per dose, with an 8-week dosing interval) were compared: a single administration of rAAV5/5Lux; sham administration followed by rAAV5/5Lux; administration of rAAV5/5EGFP followed by rAAV5/5Lux; and administration of rAAV5/5CTLA4Ig followed by rAAV5/5Lux. Animals naive to treatment served as negative controls (Fig. 4).
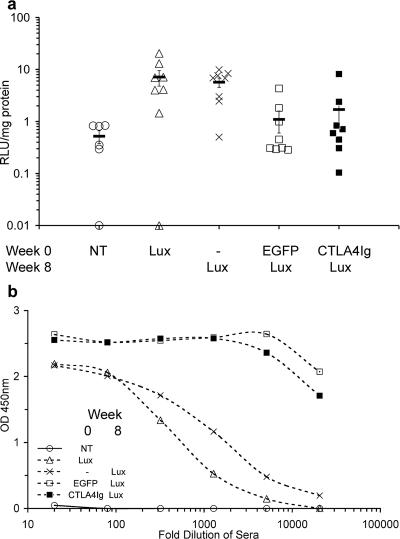
FIG. 4.
Repeat administration of rAAV5/5 to mouse nose at 8-week intervals. Female BALB/c mice (n = 6 to 8 per group) were anesthetized, and 100 μl of virus solution containing 1 × 1011 GC rAAV5/5EGFP, rAAV5/5CTLA4Ig, or rAAV5/5Lux was administered to the nose at the times indicated. Sham administration is indicated by a dash. No treatment is indicated by NT. Nasal tissue for determination of luciferase activity and blood for determination of anti-AAV5 antibodies were harvested at week 4 for the animals that received no treatment or a single administration of rAAV5/Lux and at week 20 for all other groups. (a) Nasal tissue homogenates were assayed for luciferase activity, expressed as arbitrary relative light units per mg of lung protein. The symbols (consistent for the same treatment group between panels) indicate individual mice, and the bar indicates the group mean (± SEM). (b) Serum from the blood was isolated, and pooled sera for each group were serially diluted and assayed for anti-AAV5 mouse IgG, IgM, and IgA by ELISA. OD 450nm, optical density at 450 nm.
Consistent with results of our previously published studies, delivery of a single dose of rAAV5/5 to the mouse nose resulted in significant nasal reporter gene expression (27). Similar levels of lung luciferase activity were noted in animals that received a single administration of rAAV5/5Lux and animals that received a sham administration followed by rAAV5/5Lux (7.15 ± 2.37 and 5.69 ± 1.16 RLU/mg protein, respectively; P = 0.97) (Fig. 4a). These were significantly above background levels (naive animals, 0.52 ± 0.14 RLU/mg protein; P < 0.005). Overall, animals that received either rAAV5/5EGFP or rAAV5/5CTLA4Ig were not efficiently transduced by subsequent administration of rAAV5/5Lux. Low levels of nasal luciferase activity were observed in groups of animals that received rAAV5/5EGFP followed by rAAV5/5Lux (1.1 ± 0.5 RLU/mg protein) or rAAV5/5CTLA4Ig followed by rAAV5/5Lux (1.7 ± 0.96 RLU/mg protein) and were indistinguishable from those of naive animals (0.52 ± 0.14 RLU/mg protein; P = 0.56 and P = 0.37, respectively) (Fig. 4a). It is notable that nasal luciferase activity was detectable in a proportion of animals that received two administrations of rAAV5/5 (n = 3/8 animals for rAAV5/5EGFP followed by rAAV5/5Lux, and n = 2/8 animals for rAAV5/5CTLA4Ig followed by rAAV5/5Lux). As with lung administration, nasal administration of rAAV5/5 vectors led to the generation of appreciable levels of serum anti-AAV5 antibodies, with higher titers observed for animals receiving two administrations of rAAV5/5 than for those receiving a single administration (Fig. 4b). Together these data suggest that delivery of rAAV5/5 to the nasal cavity, like delivery to the lung, leads to the generation of an inhibitory immune response.
Kinetics of anti-AAV5 antibody response after delivery of rAAV5/5 to respiratory systems of mice.
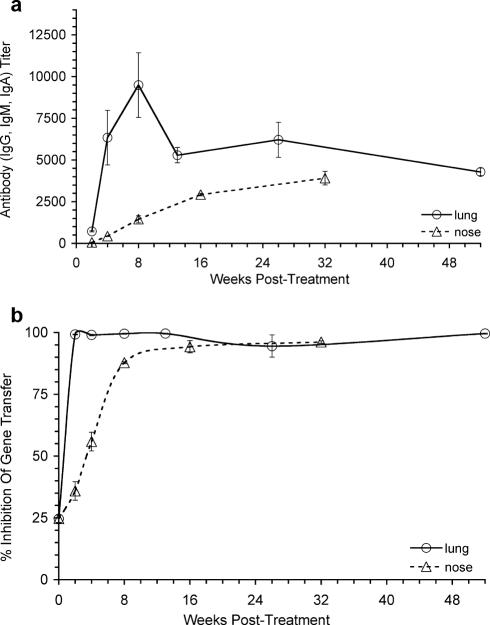
A time course of anti-AAV5 antibody activity in the serum of groups of mice after a single administration of 1 × 1011 GC of rAAV5/5Lux to either the nose or lung was established. After lung delivery, significant anti-AAV5 antibody levels were detected from 2 weeks postdelivery, rising to a maximum at 8 weeks postdelivery and then persisting at high titers to the end of the experiment at 52 weeks postdelivery (Fig. 5a). Delivery of rAAV5/5Lux to the nose led to a slower rise in antibodies, but these also persisted to the end of the experiment at 32 weeks postdelivery (Fig. 5a). Neutralizing anti-AAV5 antibody activity, which blocked rAAV5/5Lux transduction of COS7 cells in vitro, was also detected from 2 weeks postdelivery after both lung and nose delivery (Fig. 5b).
FIG. 5.
Characterization of anti-AAV5/5 antibody response after administration to mouse lungs or nose. Female BALB/c mice were anesthetized and 1 × 1011 GC rAAV5/5Lux administered to the lungs or nose. Blood was harvested at weeks 2, 4, 8, 13, 26, and 52 (for lung study) or weeks 2, 4, 8, 16, and 32 (for nose study). (a) Serum, pooled for each time point and treatment condition, was serially diluted and assayed in triplicate for anti-AAV5 mouse IgG, IgM, and IgA by ELISA. Data are presented as mean titers ± SEM. (b) Neutralizing activity in sera from the same groups was assayed by mixing rAAV5/5Lux with the pooled sera before transducing COS7 cells in vitro. The data are presented as percent inhibition (mean ± SEM) compared to transduction of COS7 cells with virus alone. The data point at 0 weeks corresponds to sera from naive mice.
Characterization of antibody responses against transgenes expressed by rAAV5/5 in repeat administration studies.
There have been reports that different transgenes can influence the immune response against rAAV particles (10). To further elucidate the components involved in generating the blocking immune response in our repeat administration studies, sera from all mice analyzed in Fig. 1 to 4 were analyzed for the presence of antitransgene antibodies by ELISA. No significant levels of antibodies were detected against any of the reporter proteins (CAT, EGFP, and Lux) (Fig. 6a to c). In contrast, high levels of anti-CTLA4Ig antibodies were detected for all mice treated with rAAV5/5CTLA4Ig (Fig. 6b and c). Overall, however, this analysis suggests that any adjuvant effect of the transgenes used here is not a significant cause for the antibody response against rAAV5 with either of the airway gene transfer models analyzed.
FIG. 6.
Antitransgene antibodies in repeat administration studies. Pooled sera from treatment regimes shown in Fig. 1, 3, and 4 (n = 5 to 8 animals per pooled group) were serially diluted and assayed in duplicate for anti-Lux, anti-CAT, anti-GFP, or anti-CTLA4Ig mouse IgG, IgM, and IgA by ELISA. Titers of antibodies were calculated, and data from two independent ELISA are shown for repeat administration of rAAV5/5 vectors expressing common reporter genes administered to the lung with an 8-week dosing interval with treatment groups described in the legend to Fig. 1 (a), expressing CTLA4Ig or luciferase to the lung with an 8-week dosing interval with treatment groups described in Fig. 3 (b), or expressing CTLA4Ig or common reporter genes to the nose with an 8-week dosing interval with treatment groups described in the legend to Fig. 4 (c). Sham administration is indicated by a dash. No treatment is indicated by NT. For comparison, anti-AAV5 antibody titers in the same sera are also shown for each group.
Successful transduction with rAAV5/5 after exposure to rAAV2/2.
Having demonstrated that a first administration of an rAAV5/5 vector largely inhibits subsequent transduction by rAAV5/5, we examined the consequences of a prior administration of an alternate AAV serotype. Three lung treatment regimes (1 × 1011 GC rAAV per dose, with an 8-week dosing interval) were compared: a single administration of rAAV5/5Lux; a single administration of rAAV2/2GFP (an AAV2-based rAAV vector analogous to rAAV5/5EGFP), and administration of rAAV2/2GFP followed by rAAV5/5Lux.
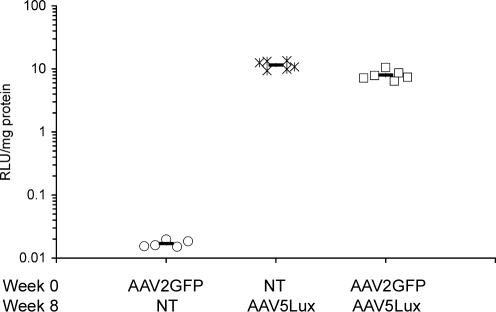
Importantly, only background levels of lung luciferase activity were noted in animals that received a single administration of rAAV2/2GFP (0.02 ± 0.001 RLU/mg protein) (Fig. 7). Crucially, levels of lung luciferase activity were significantly above background levels in mice that had received a single administration of rAAV5/5Lux (11.53 ± 0.72 RLU/mg protein; P < 0.0001) and mice that had received rAAV2/2GFP followed by rAAV5/5Lux (7.98 ± 0.59 RLU/mg protein; P < 0.0001) (Fig. 7). This confirmed that efficient repeated AAV-mediated lung transduction could be achieved by administration of rAAV vectors with differing serotypes.
FIG. 7.
Repeat administration of rAAV2/2 and rAAV5/5 to mouse lungs at 8-week intervals. Female BALB/c mice (n = 5 or 6 per group) were anesthetized and 150 μl of virus solution containing 1 × 1011 GC rAAV2/2GFP or rAAV5/5Lux was administered to the lungs at the times indicated. No treatment is indicated by NT. Lung tissue for determination of luciferase activity was harvested at week 12 for all groups. Lung tissue homogenates were assayed for luciferase activity, expressed as arbitrary relative light units per mg of lung protein. The symbols indicate individual mice; the bar indicates the group mean (± SEM).
DISCUSSION
Gene therapy for chronic inherited diseases probably requires lifelong expression of the therapeutic transgene. While several viral vectors have now demonstrated an extended duration of expression with various models, the question of immune responses against viral components needs to be addressed where repeated administration will be required in the clinical setting. With the continued interest in rAAV, especially for vectors utilizing non-AAV2 capsids, as a gene delivery vector for CF, it is timely to investigate the ability of rAAV5/5 vectors to transduce the murine respiratory system upon repeat exposure.
We first studied transduction of the mouse lung by rAAV5/5Lux after exposure to one or two doses of virus expressing different transgenes (CAT or EGFP) at 8-week or 36-week intervals. Disappointingly, Lux expression was drastically reduced in the repeat administration groups (Fig. 1a and 2). Similarly, after administration to the nose rather than the lung, delivery of rAAV5/5Lux 8 weeks after exposure to rAAV5/5EGFP did not lead to detectable Lux expression. Crucially, after even a single administration to the lungs or nose, a strong anti-rAAV5 antibody-based immune response was observed (Fig. 1b and 4b). Consistent with the reports of others, only by changing the serotype of the AAV capsid between successive administrations were we able to observe efficient rAAV transgene expression on a second administration (Fig. 7) (14, 17, 25).
A detailed time course of anti-AAV5 antibodies in sera of mice following delivery of rAAV5/5Lux to the lungs showed that a potent immune response, including neutralizing antibodies, directed against AAV5 capsid proteins is generated as early as 2 weeks postdelivery and is maintained for at least 1 year (Fig. 5). A similar antibody response was observed after nasal delivery, though this was somewhat slower in developing (Fig. 5). Such responses are consistent with the observations of others regarding the production of neutralizing antibodies against rAAV2/2 after delivery to muscle (8) or lung (16).
In other studies, transient immunosuppression using recombinant molecules such as anti-CD4, anti-CD40L, and CTLA4Ig reduced the host response to AAV and allowed efficient repeated administration in mouse muscle (23) and lungs (16). Adenoviral vectors (AdV) expressing CTLA4Ig have also successfully prevented the usual rapid clearance of AdV-transduced cells and inhibited the generation of anti-AdV antibodies with a variety of models, facilitating a successful second viral administration (18, 28). We attempted to replicate this success by examining the efficacy of rAAV5/5Lux administration after single or double prior administrations of rAAV5/5CTLA4Ig. Despite expression of CTLA4Ig from the rAAV5/5CTLA4Ig vector (Fig. 6), repeated administration was unsuccessful. Possible explanations for this failure include differences in absolute CTLA4Ig expression levels, the delay in expression noted from rAAV vectors compared with that for rAdV, possibly leaving CTLA4Ig levels too low to block antigen presentation in the critical period immediately postdelivery, or that the use of CTLA4Ig alone was insufficient.
Interestingly, while no immune reactivity against either Lux, CAT, or EGFP reporter proteins used in this study was noted, mice treated with rAAV5/5CTLA4Ig did generate anti-CTLA4Ig antibodies (Fig. 6). One possible explanation for this difference is that while the common reporter proteins reside principally in the cytoplasm of transduced cells, CTLA4Ig is engineered to be secreted (22), which may increase the likelihood of an immune response.
It is unfortunate that sourcing of recombinant CTLA4Ig was interrupted at the time of these studies, and it would be interesting to see whether this approach could improve the performance of AAV5/5 in repeat administrations. The use of recombinant CTLA4Ig and/or other potent immunomodulatory molecules may appear to be risky in the clinical setting; however, in the case of rAAV delivery, the only source of viral components is the capsid content of the inoculum. Thus, transient immunosuppression, perhaps for as little as a few days until all AAV capsid protein has been cleared from the system, could in theory be sufficient. Combined with the long-term persistence of gene transfer by rAAV vectors, this means that repeat administration may yet be feasible.
The observations we report here are limited to administration of rAAV5/5 to the nose or lungs, as our primary aim was to evaluate the possibility of multiple administrations via the airways for treatment of CF. However, since both cellular and humoral immune responses may be dependent on the route of vector administration (30), it is possible that the findings may not be directly extrapolated for other modes of administration. Furthermore, we restricted the studies presented to the common laboratory BALB/c mouse strain, and it is also possible that studies using alternate genetic backgrounds might show differing responses. It is notable that while our observations regarding a lack of efficient repeated administration after lung delivery are consistent with results of studies involving rAAV2/2, rAAV2/3, and rAAV2/6 (14, 16), published studies examining a second administration of rAAV2/5 and rAAV2/9 to C57BL/6 mice have reported more-successful results (3, 21). It was perhaps most surprising that our conclusions regarding the efficiency of repeated delivery of AAV5 capsid-containing vectors differed so starkly. Auricchio reported successful transduction of mouse lungs with rAAV2/5-βgal 5 and 6.5 months after exposure to rAAV2/5-Factor IX or rAAV2/5-hAAT, respectively, despite the presence of serum antibodies against AAV5 capsid (3). It is possible that successful repeated administration requires the generation of less than a critical threshold of anti-AAV antibodies that was not reached in the published studies but was exceeded in the studies presented here. Such a threshold might explain the modest levels of reporter gene expression noted after a second lung or nasal administration of rAAV5/5 (Fig. 1 and 4). An additional contributing factor may be the quality of AAV preparations, which could impact antibody levels. Importantly, studies of various AAV pseudotypes in mouse muscle confirm our finding that repeated exposure to vectors bearing the same capsid boosts the anticapsid antibody response (25); thus, it would be interesting to investigate the efficiencies of rAAV2/5 and rAAV2/9 vectors after more than two administrations.
In conclusion, the antibody response to AAV capsid proteins appears to remain an issue for repeated administration of rAAV5/5 to the airways of mice. Neither extending the spacing between administrations nor expressing CTLA4Ig from the virus itself allowed successful repeated administration using the delivery methods under investigation. Alternative immunosuppressive strategies may be required to allow efficient repeated rAAV5/5 administration.
Acknowledgments
We are grateful for the kind gift of plasmids pAd12, p5RC, and pAAV5LacZ from John Chiorini (National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD). We acknowledge Anne-Marie Douar, Mauro Mezzina, and their colleagues for invaluable advice on rAAV production (EuroLabCourse 2002, Génopole, Evry, France). We also thank Lee Davies, Anna Lawton, Hazel Painter, and Anusha Varathalingam for technical and methodological assistance and Eric Alton, Chris Boyd, Jane Davies, Uta Griesenbach, Tracy Higgins, and David Porteous for strategic discussions.
Our research was funded by a grant from the UK Cystic Fibrosis Trust (Bromley, United Kingdom) to the UK Cystic Fibrosis Gene Therapy Consortium (http://www.cfgenetherapy.org.uk). We acknowledge the Vector Core at the Gene Therapy Program, University of Pennsylvania (Philadelphia), for providing rAAV2/2GFP, with funding through the Vector Core Component III grant from the Cystic Fibrosis Foundation (Gene Therapy for Cystic Fibrosis and Genetic Diseases CFFS886).
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Aitken, M. L., R. B. Moss, D. A. Waltz, M. E. Dovey, M. R. Tonelli, S. C. McNamara, R. L. Gibson, B. W. Ramsey, B. J. Carter, and T. C. Reynolds. 2001. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 12:1907-1916. [DOI] [PubMed] [Google Scholar]
- 2.Auricchio, A., G. Kobinger, V. Anand, M. Hildinger, E. O'Connor, A. M. Maguire, J. M. Wilson, and J. Bennett. 2001. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum. Mol. Genet. 10:3075-3081. [DOI] [PubMed] [Google Scholar]
- 3.Auricchio, A., E. O'Connor, D. Weiner, G. P. Gao, M. Hildinger, L. Wang, R. Calcedo, and J. M. Wilson. 2002. Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Investig. 110:499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borthwick, D. W., M. Shahbazian, Q. T. Krantz, J. R. Dorin, and S. H. Randell. 2001. Evidence for stem-cell niches in the tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 24:662-670. [DOI] [PubMed] [Google Scholar]
- 5.Bouvet, M., B. Fang, S. Ekmekcioglu, L. Ji, C. D. Bucana, K. Hamada, E. A. Grimm, and J. A. Roth. 1998. Suppression of the immune response to an adenovirus vector and enhancement of intratumoral transgene expression by low-dose etoposide. Gene Ther. 5:189-195. [DOI] [PubMed] [Google Scholar]
- 6.Chiorini, J. A., F. Kim, L. Yang, and R. M. Kotin. 1999. Cloning and characterization of adeno-associated virus type 5. J. Virol. 73:1309-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 8.Chirmule, N., W. Xiao, A. Truneh, M. A. Schnell, J. V. Hughes, P. Zoltick, and J. M. Wilson. 2000. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J. Virol. 74:2420-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson, B. L., C. S. Stein, J. A. Heth, I. Martins, R. M. Kotin, T. A. Derksen, J. Zabner, A. Ghodsi, and J. A. Chiorini. 2000. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 97:3428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flotte, T. R. 2004. Immune responses to recombinant adeno-associated virus vectors: putting preclinical findings into perspective. Hum. Gene Ther. 15:716-717. [DOI] [PubMed] [Google Scholar]
- 11.Flotte, T. R., P. L. Zeitlin, T. C. Reynolds, A. E. Heald, P. Pedersen, S. Beck, C. K. Conrad, L. Brass-Ernst, M. Humphries, K. Sullivan, R. Wetzel, G. Taylor, B. J. Carter, and W. B. Guggino. 2003. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: a two-part clinical study. Hum. Gene Ther. 14:1079-1088. [DOI] [PubMed] [Google Scholar]
- 12.Gill, D. R., S. E. Smyth, C. A. Goddard, I. A. Pringle, C. F. Higgins, W. H. Colledge, and S. C. Hyde. 2001. Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1a promoter. Gene Ther. 8:1539-1546. [DOI] [PubMed] [Google Scholar]
- 13.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 14.Halbert, C. L., E. A. Rutledge, J. M. Allen, D. W. Russell, and A. D. Miller. 2000. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 74:1524-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbert, C. L., T. A. Standaert, M. L. Aitken, I. E. Alexander, D. W. Russell, and A. D. Miller. 1997. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J. Virol. 71:5932-5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halbert, C. L., T. A. Standaert, C. B. Wilson, and A. D. Miller. 1998. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J. Virol. 72:9795-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hildinger, M., A. Auricchio, G. Gao, L. Wang, N. Chirmule, and J. M. Wilson. 2001. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J. Virol. 75:6199-6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, Z., G. Schiedner, S. C. Gilchrist, S. Kochanek, and P. R. Clemens. 2004. CTLA4Ig delivered by high-capacity adenoviral vector induces stable expression of dystrophin in mdx mouse muscle. Gene Ther. 11:1453-1461. [DOI] [PubMed] [Google Scholar]
- 19.Kay, M. A., C. S. Manno, M. V. Ragni, P. J. Larson, L. B. Couto, A. McClelland, B. Glader, A. J. Chew, S. J. Tai, R. W. Herzog, V. Arruda, F. Johnson, C. Scallan, E. Skarsgard, A. W. Flake, and K. A. High. 2000. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 24:257-261. [DOI] [PubMed] [Google Scholar]
- 20.Koeberl, D. D., I. E. Alexander, C. L. Halbert, D. W. Russell, and A. D. Miller. 1997. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc. Natl. Acad. Sci. USA 94:1426-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limberis, M. P., and J. M. Wilson. 2006. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. USA 103:12993-12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linsley, P. S., P. M. Wallace, J. Johnson, M. G. Gibson, J. L. Greene, J. A. Ledbetter, C. Singh, and M. A. Tepper. 1992. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science 257:792-795. [DOI] [PubMed] [Google Scholar]
- 23.Manning, W. C., S. Zhou, M. P. Bland, J. A. Escobedo, and V. Dwarki. 1998. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum. Gene Ther. 9:477-485. [DOI] [PubMed] [Google Scholar]
- 24.Randell, S. H. 2006. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 3:718-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riviere, C., O. Danos, and A. M. Douar. 2006. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 13:1300-1308. [DOI] [PubMed] [Google Scholar]
- 26.Seiler, M. P., A. D. Miller, J. Zabner, and C. L. Halbert. 2006. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum. Gene Ther. 17:10-19. [DOI] [PubMed] [Google Scholar]
- 27.Sumner-Jones, S. G., L. A. Davies, A. Varathalingam, D. R. Gill, and S. C. Hyde. 2006. Long-term persistence of gene expression from adeno-associated virus serotype 5 in the mouse airways. Gene Ther. 13:1703-1713. [DOI] [PubMed] [Google Scholar]
- 28.Thummala, N. R., S. S. Ghosh, S. W. Lee, B. Reddy, A. Davidson, M. S. Horwitz, J. R. Chowdhury, and N. R. Chowdhury. 2002. A non-immunogenic adenoviral vector, coexpressing CTLA4Ig and bilirubin-uridine-diphosphoglucuronateglucuronosyltransferase permits long-term, repeatable transgene expression in the Gunn rat model of Crigler-Najjar syndrome. Gene Ther. 9:981-990. [DOI] [PubMed] [Google Scholar]
- 29.Wagner, J. A., A. H. Messner, M. L. Moran, R. Daifuku, K. Kouyama, J. K. Desch, S. Manley, A. M. Norbash, C. K. Conrad, S. Friborg, T. Reynolds, W. B. Guggino, R. B. Moss, B. J. Carter, J. J. Wine, T. R. Flotte, and P. Gardner. 1999. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 109:266-274. [DOI] [PubMed] [Google Scholar]
- 30.Wang, L., O. Cao, B. Swalm, E. Dobrzynski, F. Mingozzi, and R. W. Herzog. 2005. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 12:1453-1464. [DOI] [PubMed] [Google Scholar]
- 31.Xiao, X., J. Li, and R. J. Samulski. 1996. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 70:8098-8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zabner, J., M. Seiler, R. Walters, R. M. Kotin, W. Fulgeras, B. L. Davidson, and J. A. Chiorini. 2000. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 74:3852-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]