Abstract
Activating protein 2α (AP-2α) is known to be expressed in the retina, and AP-2α-null mice exhibit defects in the developing optic cup, including patterning of the neural retina (NR) and a replacement of the dorsal retinal pigmented epithelium (RPE) with NR. In this study, we analyzed the temporal and spatial retinal expression patterns of AP-2α and created a conditional deletion of AP-2α in the developing retina. AP-2α exhibited a distinct expression pattern in the developing inner nuclear layer of the retina, and colocalization studies indicated that AP-2α was exclusively expressed in postmitotic amacrine cell populations. Targeted deletion of AP-2α in the developing retina did not result in observable retinal defects. Further examination of AP-2α-null mutants revealed that the severity of the RPE defect was variable and, although defects in retinal lamination occur at later embryonic stages, earlier stages showed normal lamination and expression of markers for amacrine and ganglion cells. Together, these data demonstrate that, whereas AP-2α alone does not play an intrinsic role in retinogenesis, it has non-cell-autonomous effects on optic cup development. Additional expression analyses showed that multiple AP-2 proteins are present in the developing retina, which will be important to future studies.
The retina is an extension of the central nervous system derived from the forebrain neural ectoderm. During vertebrate eye development, the diencephalon evaginates to form optic vesicles, which subsequently invaginate to form a bilayered optic cup. The inner layer of the optic cup will give rise to the neural retina (NR), and the outer layer becomes the retinal pigmented epithelium (RPE) (13). Six principal types of neurons and the Müller glia cells that comprise the NR are generated in a fixed, overlapping chronological order (69). Ganglion cells are “born” (i.e., become postmitotic) first, followed by amacrine, horizontal, and cone photoreceptor cells, and ending with bipolar and Müller glia cells. The birth of rod photoreceptors spans nearly the entire period of retinal histogenesis, which begins at embryonic day 10.5 (E10.5) in mice and continues for approximately 3 weeks, ending at postnatal day 11 (P11) (69). A “central-to-peripheral” gradient of differentiation has been described in the NR, where the genesis of a particular cell type begins in the central retina (near the optic nerve head) and spreads toward the peripheral retina (next to the ciliary body) (26, 37, 47).
A range of extrinsic and intrinsic factors control the many steps that retinal progenitor cells (RPCs) progress through during development, including cell cycle exit, cell fate bias or commitment, and differentiation into a functional neuron or glial cell. The prevailing model to explain how different retinal cell fates are determined from multipotent progenitors suggests that RPCs progress through states of competence, in which their continually changing intrinsic properties determine how they will respond to external signals at given times during development (10, 11, 32). There is increasing evidence for homeodomain (HD) and basic helix-loop-helix (bHLH) transcription factors as intrinsic regulators of retinal progenitor maintenance, cell fate determination, and terminal differentiation (10, 29, 32, 33). In the early optic cup, the majority of RPCs coexpress a core set of transcription factors that includes the HD factors Pax6, Six3, and Chx10 and the bHLH factor Hes1 (33, 35). As retinogenesis progresses and the transcriptional programs of RPCs begin to diverge, expression of these core factors becomes restricted to select cell lineages, while additional transcription factors are upregulated in subsets of RPCs (33, 35).
The amacrine cells are the most diverse class of retinal neurons, with more than 20 and possibly as many as 40 to 50 subtypes (36, 55, 59). Much remains to be determined about the intrinsic regulation of amacrine cell development. The bHLH factors Math3 and NeuroD were shown to have redundant roles in amacrine cell determination, illustrated by an absence of amacrine cells in Math3-NeuroD double-mutant retinas (27), but normal amacrine cell development in single Math3 or NeuroD knockouts (40, 56). The forkhead/winged helix transcription factor Foxn4 has recently been added to the amacrine cell transcriptional network, as demonstrated by its promotion of amacrine cell formation through regulation of Math3 and NeuroD expression (31). Two HD factors, Pax6 and Barhl2, were shown to influence the development of the glycinergic amacrine cell population (34, 39). Interestingly, conditional deletion of Pax6 from the mouse retina prevents the genesis of all cell types except amacrine cells; however, the resulting amacrine cell population is nearly devoid of the glycinergic class (34). The list of amacrine cell factors that control the development of this diverse type of neuron, however, is far from complete.
Activating protein 2α (AP-2α) is another transcription factor previously shown to be expressed in chick amacrine cells (3). The AP-2 transcription factors are a family of retinoic acid-responsive proteins that have been shown to play essential roles in development by regulating the differentiation, proliferation, and survival of cells (see references 17 and 24 for reviews). In mice and humans, the AP-2 family of proteins is encoded by five separate genes (named Tcfap2a to Tcfap2e in mice) which exhibit both overlapping and unique developmental expression patterns in derivatives of the neural crest and surface ectoderm (including the lens and cornea), renal and urogential tissues, limb buds, and structures of the central nervous system, including the retina (8, 18, 38, 41-43, 63, 64, 71). In the carboxy-terminal half of the protein, all family members share a basic DNA-binding region, which is followed by a helix-span-helix dimerization motif through which the AP-2 proteins can form homodimers, or heterodimers with other family members (18, 41, 44, 65, 66, 71). Both AP-2α and AP-2β have been linked to fatty acid-related ocular development (3, 4, 72). During chick retinogenesis, AP-2α and AP-2β have been shown to act as transcriptional repressors of retinal fatty acid binding protein (R-FABP), a homologue of mammalian brain FABP that is strongly expressed in the developing chick retina (3, 4). Also, pregnant mice treated with a retinoic acid receptor antagonist produce offspring with ocular defects (including retinal abnormalities), as well as a reduction in ocular AP-2 expression seen most prominently in the retina and eyelids (72). Through ectopic expression and germ line and tissue-specific knockout (KO) studies, we have previously demonstrated roles for AP-2α in development of the lens and cornea (16, 62, 63). The AP-2α germ line null mice exhibited a variety of ocular abnormalities, including defects in the developing optic cup (63). Given the severe phenotype of these mice and the fact that AP-2α was missing from all tissues, it was not clear whether the optic cup defects were secondary and possibly caused by a loss of inductive signals from other ocular or extraocular tissues. Thus, the intrinsic role of AP-2α in retinogenesis remained to be explored.
The aims of the present study were twofold. First, a detailed temporal and spatial analysis of AP-2α expression in the developing mouse retina was conducted. Second, the requirement of AP-2α in retinogenesis was investigated using the Cre-loxP system to create a conditional deletion of Tcfap2a in the developing retina. Our findings revealed that AP-2α is exclusively expressed in postmitotic amacrine cells during retinal development and in mature amacrine cells in the adult retina. AP-2α protein was detected in a range of amacrine cell subpopulations in the inner nuclear layer (INL) and ganglion cell layer (GCL). Detailed examination of the conditional KO mice revealed no detectable abnormalities in retinal lamination or patterning. Further examination of optic cup defects in the AP-2α-null mutants revealed that defects in retinal lamination occur at late embryonic stages and the severity of the phenotype, specifically the RPE defect, is variable. Together, these data suggest that the retinal phenotype of AP-2α-null mutants resulted from lack of the appropriate tissue-tissue interactions rather than intrinsic loss of AP-2α, demonstrating non-cell-autonomous roles for AP-2α in optic cup development. Further expression studies showed that additional AP-2 family members are expressed in the developing retina, which will be important to consider during future investigations.
MATERIALS AND METHODS
Generation of AP-2α conditional KO mouse and AP-2α-null mutant.
All animal procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Using a mating scheme with two crosses, Re-AP-2α mutants with Tcfap2a conditionally deleted from the developing retina were created as follows. In the first cross, mice already heterozygous for the Tcfap2a:LacZ KI-null allele (due to a germ line lacZ knock-in insertion disrupting exon 7 [7]; Tcfap2aki7lacZ/+) were mated with heterozygous α-Cre+/− transgenic mice expressing Cre recombinase under control of the “Pax6 α enhancer,” a retina-specific regulatory element of the murine Pax6 gene [Tg(Pax6-cre, GFP)2Pgr; MGI:3052668] (30, 34). The chosen progeny, which contained the α-Cre transgene while also maintaining the Tcfap2aki7lacZ/+ genotype (Tcfap2aki7lacZ/+/α-Cre+/−), were then crossed with mice homozygous for the Tcfap2alox allele (i.e., mice with both Tcfap2a alleles flanked by loxP sites; Tcfap2alox/lox) (6). The desired Re-AP-2α mutant offspring contained the α-Cre transgene, one Tcfap2alox allele, and one Tcfap2aki7lacZ-null allele (Tcfap2aki7lacZ/lox /α-Cre+/−). Upon activation at E10.5 by the Pax6 retina-specific regulatory element driving its expression (34), Cre recombinase acts on the loxP sites to excise the Tcfap2alox allele, creating the shortened, nonfunctional Floxdel allele. As mice were mated to generate mutants, the Tcfap2a alleles and presence of the α-Cre transgene were monitored by PCR. Noon on the day of vaginal plug detection was considered day 0.5 (E0.5) of embryogenesis. DNA was extracted from embryonic tail samples or adult ear clips by using the DNeasy tissue kit (QIAGEN). Mouse genotypes were determined by well-established and previously reported PCR protocols (7). Mice carrying the α-Cre transgene were identified by using the primers Cre1 (5′-GCT GGT TAG CAC CGC AGG TGT AGA G-3′) and Cre3 (5′-CGC CAT CTT CCA GCA GGC GCA CC-3′) that correspond to nucleotides 1090 to 1114 and nucleotides 1489 to 1511 of the Cre recombinase gene, respectively. PCR analysis was performed for 35 cycles (45 s at 95°C, 45 s at 67°C, and 1.5 min at 72°C), generating a 420-bp fragment. To detect the Tcfap2aki7lacZ allele, PCR genotyping was performed using the forward primer Alpha 6/7 (5′-GAA AGG TGT AGG CAG AAG TTT GTC AGG GC-3′) and reverse primers Alpha3′KO (5′-CGT GTG GCT GTT GGG GTT GTT GCT GAG GTA C-3′) and IRESUP (5′-GCT AGA CTA GTC TAG CTA GAG CGG CCC GGG-3′) for 35 cycles (45 s at 95°C, 45 s at 70°C, and 1 min at 72°C), generating a 500-bp wild-type (Tcfap2a+) product and a 300-bp Tcfap2aki7lacZ product. PCR was also used to confirm retina-specific excision of the Tcfap2alox allele. DNA was extracted from tail, brain, eyelid, and retinal tissue and analyzed for an intact or recombined Tcfap2alox allele using the primers Alflp (5′-CCT GCC TTG GAA CCA TGA CCC TCA G-3′), Alflox4 (5′-CCC AAA GTG CCT GGG CTG AAT TGA C-3′), and Alfscsq (5′-GAA TCT AGC TTG GAG GCT TAT GTC-3′). PCR was performed for 30 cycles (45 s at 95°C, 45 s at 65°C, and 1.5 min at 72°C). This reaction generated a 490-bp product for the Tcfap2aki7lacZ allele, a 560-bp product for the intact Tcfap2alox allele, and a 185-bp product for the excised and recombined Tcfap2alox (Floxdel) allele. The primers that detect the Tcfap2alox allele (Alflox4 and Alfscsq) anneal outside of the LacZ insertion, and therefore will also generate a 490-bp product on control mice with one Tcfap2a+ allele. Littermates that contained two functional copies of Tcfap2a (one Tcfap2a+ and one Tcfap2alox, which does not affect the AP-2α protein product [6]; Tcfap2alox/+) and lacked the α-Cre transgene were used as controls. To generate AP-2α germ line null mutants, two mice heterozygous for the Tcfap2aki7lacZ-null allele (Tcfap2aki7lacZ/+) were mated, and genotypes were monitored by PCR using the primers Alpha 6/7, Alpha3′KO, and IRESUP as described above. Mutants were homozygous for the Tcfap2aki7lacZ-null allele (Tcfap2aki7lacZ/ki7lacZ), and littermates used as controls were homozygous for the wild-type Tcfap2a+ allele (Tcfap2a+/+). The JAX GEMM ROSA26 Cre reporter strain of mice [B6.129S4-Gt(ROSA)26Sortm1Sor/J; The Jackson Laboratory stock no. 003474] was also used. Homozygous breeding pairs were purchased and used to maintain this line; therefore, genotyping was not required.
Histology.
Whole embryos/neonates or dissected whole eyes were collected from mice euthanized by CO2 overdose. Tissue was either fixed in 10% neutral buffered formalin (Sigma-Aldrich, Oakville, Ontario, Canada) overnight (at 4°C for eyes or room temperature for embryos), processed, and embedded in paraffin or fresh-frozen in Tissue-Tek OCT (Sakura-Finetek, Torrance, CA). Serial sections were cut 4 or 5 μm in thickness and used for immunofluorescence analysis or hematoxylin and eosin (H&E) staining; frozen sections used for X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining were cut 12 μm thick. For all stages examined, a sample size of between three and six retinas were stained (either H&E or immunofluorescence) for each genotype (control or mutant). For the cell counting studies (see below), sample sizes of three mutant and three control retinas were used for each strain.
Immunofluorescence.
Indirect immunofluorescence was performed with the following primary antibodies: mouse monoclonal anti-AP-2α (3B5) used undiluted (developed by Trevor Williams, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); rabbit polyclonal anti-AP-2α at 1:800 (Abcam, Cambridge, MA); goat polyclonal anticalretinin at 1:800 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); rabbit polyclonal anti-Islet-1&2 (K5), which detects both the Islet-1 and Islet-2 proteins, at 1:500 (Thomas Jessell laboratory, Columbia University, New York, NY); goat polyclonal anti-Brn3b at 1:200 (Santa Cruz); mouse monoclonal anti-syntaxin-1 at 1:2,000 (Sigma); goat polyclonal anti-glycine transporter 1 (anti-GlyT1) at 1:5,000 (Chemicon International, Inc., Temecula, CA); rabbit polyclonal anti-γ-aminobutyric acid (GABA) transporter 1 (anti-GAT-1) at 1:250 (Abcam); rabbit polyclonal phospho-histone H3 (PH3) at 1:30 (Upstate, Charlottesville, VA); rabbit polyclonal Ki67 at 1:1,000 (Novocastra, Vision BioSystems, Norwell, MA); mouse polyclonal AP-2β at 1:400 (Abnova Corp., Taipei City, Taiwan); and rabbit polyclonal anti-AP-2β at 1:50 (Cell Signaling Technology, Inc., Danvers, MA). Fluorescent secondary antibodies were either fluorescein isothiocyanate or rhodamine (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) always used 1:50 for 1 h at room temperature) or Alexa Fluor 568 (Invitrogen/Molecular Probes, Burlington, Ontario, Canada) used at 1:200 for 1 h at room temperature. Paraffin-embedded sections were deparaffinized in xylene, hydrated (through 100, 95, and 70% ethanol, followed by water), treated with 10 mM sodium citrate buffer (pH 6.0; boiling for 20 min) for antigen retrieval, blocked with normal serum, and incubated with primary antibodies overnight at 4°C. For all colocalizations, both primaries and both secondaries were mixed and incubated simultaneously. All stains were mounted with Vectashield mounting medium containing 4,6-diamino-2-phenylindole (DAPI; Vector Laboratories, Burlington, Ontario, Canada). Each staining experiment included a negative control with no primary antibody. Single-antibody controls were also used for all colocalization studies. All staining was visualized with a microscope (Leica, Deerfield, IL) equipped with an immunofluorescence attachment, and images were captured with a high-resolution camera and associated software (Open-Lab; Improvision, Lexington, MA). Images were reproduced for publication with image-management software (Photoshop 7.0; Adobe Systems, Inc., Mountain View, CA).
Fluorogold labeling.
Adult mice were anesthetized by intraperitoneal injection of ketamine-xylazine at a dose of 0.1 ml/10 g (body weight) and placed in a stereotactic device. The skull was exposed, and bregma was located. The coordinates of the superior colliculi in the left and right hemispheres were determined from a stereotactic mouse brain atlas, and holes were made at these positions. The retrograde neurotracer dye Fluorogold was stereotactically applied (1.5 μl in each hemisphere) using a Hamilton syringe, and the skin over the wound was sutured. Mice were rehydrated with a saline solution immediately after surgery and placed on a heating pad during recovery. Mice were euthanized by CO2 overdose 48 h later, and whole eyes were removed, fixed in 10% neutral buffered formalin overnight at 4°C, processed, paraffin embedded, and cut at 4 μm. Fluorogold-labeled retinal ganglion cells were visualized on sections costained for AP-2α as follows. Given that the antigen retrieval necessary to detect AP-2α protein expression destroys Fluorogold fluorescence, sections were first deparaffinized and hydrated, and the Fluorogold pictures were taken under the DAPI filter prior to immunostaining. Sections were subsequently stained with the anti-AP-2α antibody as described above.
X-Gal staining.
Activity of retina-specific Cre recombinase was confirmed by mating α-Cre+/− mice with the ROSA26 Cre reporter strain (53). The ROSA26 strain contains the lacZ gene driven by a constitutively active promoter; however, the lacZ gene is interrupted by a neo cassette flanked by loxP sites (53). Thus, lacZ expression will occur upon removal of the floxed intervening segment by Cre recombinase. The resulting offspring were genotyped for the presence of the α-Cre gene. Horizontal cryosections of E13.5 and P2 α-Cre+/−/ROSA26+/− or α-Cre−/−/ROSA26+/− mouse heads were fixed in cold formalin for 10 min, washed three times in phosphate-buffered saline (PBS), and rinsed in distilled water. X-Gal dilution buffer (5 mM potassium ferricyanide crystalline, 5 mM potassium ferricyanide trihydrate, and 2 mM magnesium chloride in 1× PBS) was warmed to 37°C and used to dilute (1:40) the X-Gal stock solution (4% X-Gal in N,N-dimethylformamide) to create an X-Gal working solution. The warmed X-Gal working solution was applied directly to the slides, which were incubated overnight at 37°C. Slides were rinsed in PBS, dehydrated in an ethanol series (70, 95, and 100%), and then placed in xylene before mounting with Permount (Fisher Scientific, Pittsburgh, PA).
Cell counting.
Horizontal 4-μm sections containing the optic nerve from E15.5 mutant and control littermates were stained with anticalretinin and anti-Islet-1&2 antibodies as described above. After staining, sections were partitioned such that the nasal side of the retina was separated into four bins (bins 1 to 4) by dividing the ventricular edge into equal parts and extending a line to the vitreal edge, as described in a number of other studies (9, 12, 15). DAPI-stained nuclei and cells immunoreactive for calretinin or Islet-1&2 were then counted in bins 1 and 2, the two peripheral-most bins that include the peripheral half of the retina. Counts were expressed as a percentage of DAPI-positive cells (shown as the mean ± the standard error for three retinas). The Student t test was used for statistical analysis. Counting was performed in bins 1 and 2 to ensure that AP-2α was deleted from the counted regions. Sections closely preceding or following those used for counting were stained with anti-AP-2α to confirm AP-2α deletion in each mutant retina.
ISH.
The preparation of the sense and antisense in situ hybridization (ISH) probes for AP-2α, AP-2β, AP-2δ, AP-2γ, and AP-2ɛ has been described previously (18). Dissected embryos or whole eyes were fixed, paraffin embedded, and sectioned as described above using 0.1% diethyl pyrocarbonate in the water bath to preserve RNA. A standard ISH protocol (52) was used with minor modifications. Briefly, digoxigenin-labeled sense or antisense RNA probe was incubated with the tissue sections overnight at 70°C in hybridization buffer. After washing to remove unhybridized RNA probe, alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche) was used to detect the digoxigenin-labeled RNA probe. Colorimetric detection was performed with BM purple AP substrate (Roche). The slides were counterstained with Nuclear Fast Red. Images were captured and prepared as described above.
RESULTS
Expression pattern of AP-2α in the developing and adult retina.
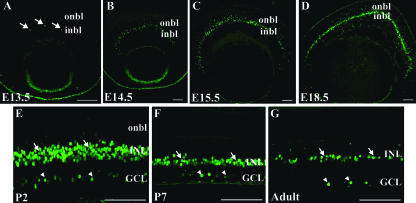
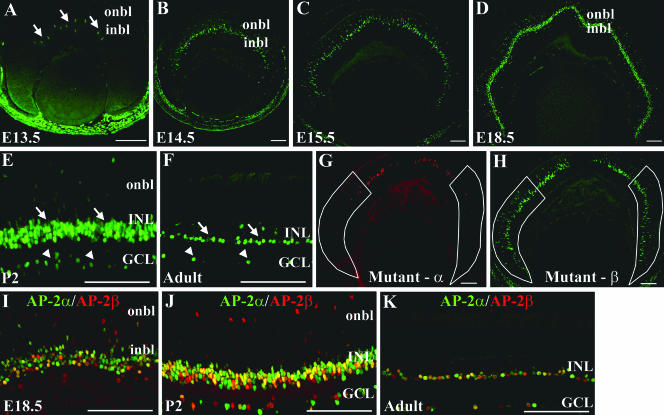
The pattern of AP-2α protein expression was assessed by using immunofluorescence with a monoclonal anti-AP-2α antibody. At E13.5, AP-2α protein was detected in multiple ocular tissues, including the anterior lens epithelium, corneal epithelium, and NR tissue of the optic cup, as previously reported (63). AP-2α was initially detected in a few nuclei of cells of the inner neuroblast layer, or presumptive INL, in the central retina (Fig. 1A). Between E13.5 and E15.5, the number of AP-2α-positive cells in the INL increased and spread to the peripheral retina (Fig. 1B and C) in a pattern that corresponds to the central-to-peripheral gradient of cell differentiation during retinogenesis. AP-2α continued to be strongly expressed in the inner portion of the developing INL throughout embryogenesis (Fig. 1D), where differentiating amacrine cells are located. Around the time of birth, its expression was first detected in a subset of cells in the GCL and also remained in the INL (Fig. 1E). This pattern persisted throughout the rest of postnatal retinogenesis and was also maintained in the adult (Fig. 1F and G). At no time was AP-2α detected in the RPE.
FIG. 1.
Expression pattern of the AP-2α protein in the developing mouse retina. Paraffin-embedded sections of embryos or dissected eyes were sectioned at 4 to 5 μm and stained with anti-AP-2α. (A) At E13.5, AP-2α initially appears in the central retina, in cells of the inner neuroblast layer (or presumptive INL; arrows). (B to D) As embryogenesis progresses, AP-2α expression extends to the periphery in a band of cells that is largely confined to the developing INL. (E to G) By P2 and continuing throughout adulthood, it is expressed in the INL (arrows), as well as a subset of cells in the GCL (arrowheads). i/onbl, inner/outer neuroblast layer. Scale bars, 100 μm.
AP-2α is not expressed in mitotic cells in the developing retina.
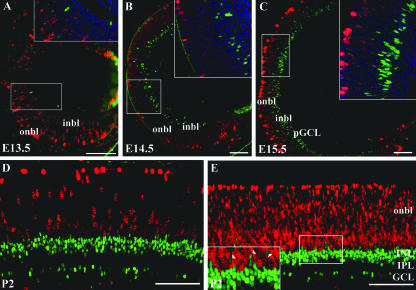
Developmentally important transcription factors that are expressed in proliferating RPCs are more likely to be involved in earlier aspects of retinal development, such as the generation of a “competence state.” If expressed in “postmitotic transition cells” (which have completed their terminal M phase but are not yet fully differentiated), transcription factors are more likely to be involved in later aspects of retinogenesis, such as final cell fate commitment or differentiation. To determine whether any AP-2α-positive cells were mitotic or whether AP-2α was expressed exclusively in postmitotic cells, embryonic and postnatal eyes were double immunostained for AP-2α and two different markers of proliferation: PH3, which exhibits a distinctive punctuate staining pattern in nuclei of mitotic cells, and the nuclear antigen Ki67, which is expressed by cells in all phases of the cell cycle (G1, S, G2, and M). The key embryonic stages for AP-2α initial expression and central-to-peripheral expansion (E13.5 to E15.5) were examined, along with a later postnatal stage (P2). At E13.5 (when the first AP-2α-positive retinal cells appear), cells expressing AP-2α did not express PH3 (Fig. 2A) or Ki67 (data not shown). Furthermore, for the remainder of the stages of retinogenesis examined, AP-2α-positive cells did not colocalize with either proliferation marker (Fig. 2B-E and not shown), including the small number of AP-2α-positive cells scattered in the highly proliferative outer neuroblast layer. These findings suggest that AP-2α is exclusively expressed in postmitotic transition cells.
FIG. 2.
AP-2α is not expressed in mitotic cells. Paraffin 4-μm horizontal sections from each developmental stage were double immunostained with anti-AP-2α (green) and the proliferation markers anti-phospho-histone H3 (A to D) or anti-Ki67 (E), both in red. No double-stained cells were detected in the retinas of embryonic (A to C) or neonatal (D and E) eyes. Squared areas are magnified at inset (top right; shown with nuclear stain DAPI [blue]). Arrowheads in panel E denote AP-2α-positive cells scattered in the highly proliferative outer neuroblast layer, which do not colocalize with Ki67. i/onbl, inner/outer neuroblast layer; pGCL, presumptive ganglion cell layer. Scale bars, 100 μm.
AP-2α is exclusively expressed in amacrine cell populations.
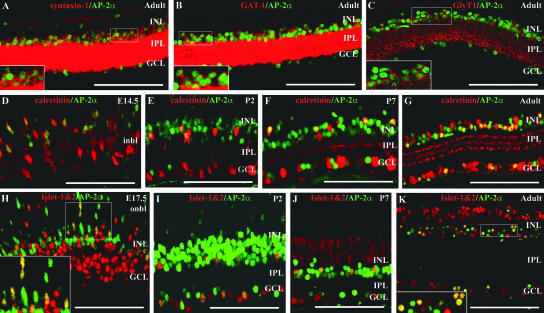
The location of AP-2α-positive cells in the inner half of the INL and a subset of cells in the GCL suggested that AP-2α expression may be confined exclusively to developing and mature amacrine cells. To investigate this hypothesis, a series of immunofluorescence colocalization studies were conducted, in which developing and mature retinas were double stained for AP-2α and markers of different retinal cell types (Fig. 3). Colocalizations were performed for AP-2α and three different amacrine cell membrane markers (syntaxin-1, GAT-1, and GlyT1) that are reliable indicators of mature amacrine interneurons. All three markers label amacrine cell bodies and processes in the inner plexiform layer (IPL) (Fig. 3A to C). The pan-amacrine marker syntaxin 1 is a synaptic vesicle protein that labels amacrine and horizontal cells in the rodent retina (1). In adult mice, syntaxin-1-positive amacrine cell membranes could be seen surrounding AP-2α-positive nuclei (Fig. 3A). The majority of amacrine cells in the mammalian retina contain either glycine or GABA inhibitory neurotransmitters, making glycinergic and GABAergic the two main amacrine cell populations, with each comprising close to half of all amacrine cells (59). AP-2α protein was found to be expressed in both glycinergic and GABAergic amacrine cells. Double stains with anti-AP-2α and anti-GlyT1 or anti-GAT-1 antibodies were similar to that of syntaxin-1, in that GlyT1-positive or GAT-1-positive membranes were found to surround AP-2α-positive nuclei (Fig. 3B and C). Double stains were also performed for AP-2α and two early activated inner retina markers, calretinin and Islet-1&2. We examined the key stages for AP-2α central-to-peripheral expansion (E13.5 to E15.5), as well as additional stages in the developing and mature retina. Starting at E14.5, a large proportion of AP-2α-positive cells were also immunoreactive for calretinin, a calcium-binding protein that labels amacrine and ganglion cells in rodents (Fig. 3D). By P2, and continuing through P7 and in the mature retina, a subset of AP-2α-positive cells colocalized with a proportion of cells immunoreactive for calretinin (Fig. 3E to G). Starting at E15.5, double-stained cells were also observed when AP-2α was colocalized with an antibody for Islet-1&2 (Fig. 3H to K and data not shown). The Islet-1 and Islet-2 transcription factors are members of the developmentally important LIM HD gene family (58). In the mouse retina, Islet-2 is an early marker for a subpopulation of ganglion cells (45), and Islet-1 labels amacrine, bipolar, and ganglion cells (19, 23). Islet-1 expression in amacrine cells is specific to the cholinergic population and provides an early cholinergic amacrine cell marker that precedes choline acetyltransferase immunoreactivity (19). A subset of AP-2α-positive cells colocalized with Islet-1-positive amacrine cells in the inner INL but not with Islet-1-positive bipolar cells, which can be easily distinguished from amacrine cells based on their location in middle to outer portions of the INL (Fig. 3J to K). Double-stained cells were also observed in the GCL, which could correspond to either Islet-1 or Islet-2-positive cells. Together, these results demonstrate that AP-2α is expressed in all subpopulations of amacrine cells examined in the INL and GCL and is not expressed in other cell types whose nuclei reside in the INL (bipolar and horizontal cells).
FIG. 3.
AP-2α colocalizes with syntaxin-1, GAT-1, GlyT1, calretinin, and Islet-1&2. Paraffin sections (4 to 5 μm) at different embryonic or postnatal stages were double stained with anti-AP-2α (green) and either anti-syntaxin-1, GlyT1, GAT-1, calretinin, or Islet-1&2 antibodies (red). A subset of AP-2α-positive cells colocalized with cells immunoreactive for syntaxin-1 (A), GAT-1 (B), GlyT1 (C), calretinin (D to G), and Islet-1&2 (H to K). Boxed areas in panels A to C, H, and K are magnified at the bottom left. i/onbl, inner/outer neuroblast layer. Scale bars, 100 μm.
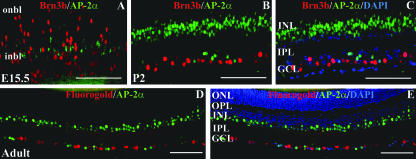
Since some of the markers with which AP-2α colocalized in the GCL (calretinin and Islet-1&2) have been shown to label ganglion cells in addition to amacrine cells, it was still unclear whether the AP-2α-positive cells in the GCL were displaced amacrine cells, ganglion cells, or a combination of both. To further investigate AP-2α expression in the GCL, eyes were double stained for AP-2α and Brn3b, a POU domain transcription factor expressed exclusively in ganglion cells in the mouse retina (21, 67, 68). In addition, to substantiate the Brn3b results, retinal ganglion cells were retrogradely labeled by injecting the fluorescent tracer Fluorogold into the superior colliculus of an adult mouse. These eyes were then sectioned and stained for AP-2α. Neither Brn3b-positive ganglion cells (Fig. 4A to C) nor Fluorogold-labeled ganglion cells (Fig. 4D and E) colocalized with AP-2α-positive cells. Thus, AP-2α-positive cells detected in the ganglion cell layer are likely to be displaced amacrine cells.
FIG. 4.
AP-2α is not expressed in retinal ganglion cells. (A to C) Paraffin sections (4 μm) of E15.5 embryos and P2 eyes were double stained with anti-AP-2α (green) and anti-Brn3b (red). (B and C) Same section, shown with or without the nuclear stain DAPI (blue). (D to E) Fluorogold-labeled whole eyes were formalin fixed, paraffin embedded, and sectioned at 4 μm. The antigen retrieval necessary to detect AP-2α (shown in green) destroys Fluorogold fluorescence (shown in red); therefore, Fluorogold pictures were taken under the DAPI filter prior to immunostaining. (D and E) Same section, shown with or without DAPI-stained nuclei (blue). i/onbl, inner/outer neuroblast layer; ONL, outer nuclear layer; OPL, outer plexiform layer. Scale bars, 100 μm.
Effect of retina-specific deletion of AP-2α during retinogenesis.
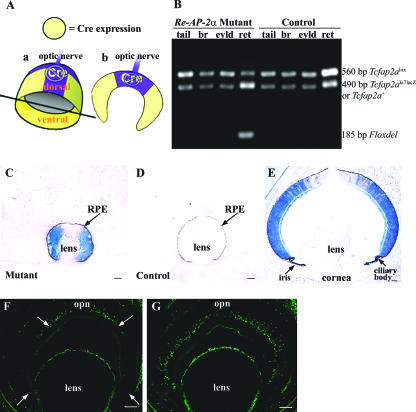
The Cre-loxP system was used to conditionally delete AP-2α from the developing retina. The “floxed” allele (Tcfap2alox) has loxP sites flanking the fifth and sixth exons of the AP-2α gene (6), and the α-Cre transgenics express Cre recombinase under control of the Pax6 α enhancer, a retina-specific regulatory element of the murine Pax6 gene (30, 34). The expression pattern directed by the Pax6 α enhancer has been previously characterized and was shown to occur in the peripheral aspects of the nasal and temporal NR but not in the central (i.e., toward the optic nerve head) and dorsal NR or the RPE (2, 30, 34) (Fig. 5A). PCR analysis confirmed that the Cre-mediated excision of Tcfap2a was retina specific and did not occur in any nonretinal tissues, including the eyelid, brain, and tail (Fig. 5B). To monitor Cre recombinase activity, α-Cre mice were bred with the ROSA26 Cre reporter strain (53), in which lacZ expression will occur upon removal of a floxed intervening segment by Cre recombinase. X-Gal staining of E13.5 and P2 heads showed that Cre activity was confined to the peripheral nasal and peripheral temporal retina (also extending into the ciliary body and iris, as previously shown (34), and was not observed in the RPE or any other tissues (Fig. 5C to E). Deletion of the AP-2α protein was confirmed by immunofluorescence with the anti-AP-2α antibody, as shown for an E15.5 Re-AP-2α mutant and control littermate (Fig. 5F and G). Thus, the pattern of Cre recombinase activity in α-Cre mice resulted in AP-2α deletion in the peripheral nasal and peripheral temporal NR, while some AP-2α expression remained in the central and dorsal retina. Previous studies with this α-Cre transgenic mouse strain have reported the same deletion pattern of the Pax6, sonic hedgehog (Shh), and retinoblastoma (Rb) genes (12, 34, 61).
FIG. 5.
Cre recombinase activity and AP-2α deletion are specific to the peripheral retina. (A) Cre recombinase expression in α-Cre mice occurs in the peripheral aspects of the nasal and temporal NR but not in the central (i.e., toward the optic nerve head) and dorsal NR. Thus, cutting the retina horizontally at the optic nerve (in plane shown in subpanel a) results in sections (subpanel b) with Cre expression in the peripheral but not central retina. (B) Excision of Tcfap2a gene in mutant retina shown by PCR. Genomic DNA from the whole retina was examined by using primers Alflp, Alflox4, and Alfscsq (6) on Re-AP-2α mutants and control littermates. Tissues that do not express Cre recombinase display the 560-bp undeleted Tcfap2alox allele and a 490-bp product that represents either the Tcfap2aki7lacZ-null allele (in mutants) or a Tcfap2a+ (wild-type) copy (in littermates chosen as controls). The Tcfap2aki7lacZ and Tcfap2a+ alleles are distinguished in a separate PCR used for genotyping (not shown). In retinas of Re-AP-2α mutants, Cre recombinase acts on the loxP sites of the Tcfap2alox allele to excise the Tcfap2a gene, producing a shortened 185-bp “Floxdel” PCR product. Note that the Re-AP-2α mutants still contain a fraction of the unexcised Tcfap2alox allele due to the fact that Cre is not expressed throughout the entire retina. (C to E) α-Cre mice were bred with the ROSA26 Cre reporter strain (53), in which lacZ expression will occur upon removal of a floxed intervening segment by Cre recombinase. Horizontal sections of E13.5 (C and D) and P2 (E) heads were stained with X-Gal to detect lacZ activity, confirming the expected Cre activity in the peripheral retina. Black arrows denote the location of the RPE in E13.5 mutant and control. (F and G) Horizontal sections (at the optic nerve) of an E15.5 Re-AP-2α mutant (F) and a control littermate (G) stained with the anti-AP-2α antibody confirm regions lacking the AP-2α protein (white arrows) in the peripheral retina. br, brain; eyld, eyelid; ret, retina; opn, optic nerve. Scale bars, 100 μm.
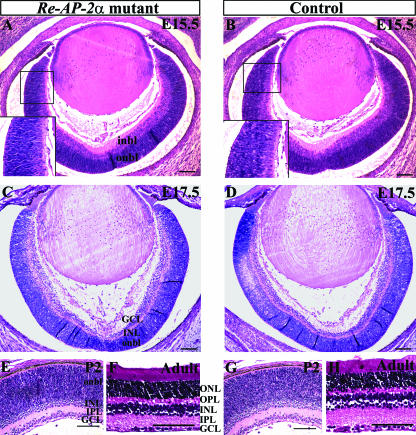
H&E staining of embryonic and postnatal horizontal sections showed that the Re-AP-2α retinas resembled control retinas at the morphological level (Fig. 6). We examined two embryonic stages that occur directly following the central-to-peripheral expansion of AP-2α expression, as well as a developing postnatal and adult stage to examine later development. At E15.5 (when large numbers of amacrine and ganglion cells are forming) the Re-AP-2α retinas appeared identical to their corresponding control retinas (Fig. 6A and B), with the expected appearance of a thick neuroblast layer, followed by an emerging GCL that has been shown to contain developing amacrine cells as well (25). At E17.5 the Re-AP-2α retinas remained morphologically comparable to controls (Fig. 6C and D), with both containing a newly distinguishable IPL comprised of amacrine and ganglion cell processes. By P2, the Re-AP-2α retinas continued to resemble those of their control littermates, with discernible amacrine cells adjacent to the IPL (Fig. 6E and G). These histological examinations indicated that the size and shape of the Re-AP-2α retinas were not altered compared to controls, and overall lamination appeared to proceed identically in Re-AP-2α and control retinas, resulting in fully laminated adult retinas (Fig. 6F and H) with all layers intact.
FIG. 6.
Histology of Re-AP-2α and control retinas. H&E staining on 5-μm horizontal paraffin sections of E15.5 (A and B), E17.5 (C and D), P2 (E and G), and adult (F and H) Re-AP-2α mutants and control littermates. The sizes, shapes, and lamination of Re-AP-2α retinas resembled those of controls at all stages examined. Boxed areas in panels A and B are magnified in the insets (bottom left). i/onbl, inner/outer neuroblast layer; ONL, outer nuclear layer; OPL, outer plexiform layer. Scale bars, 100 μm.
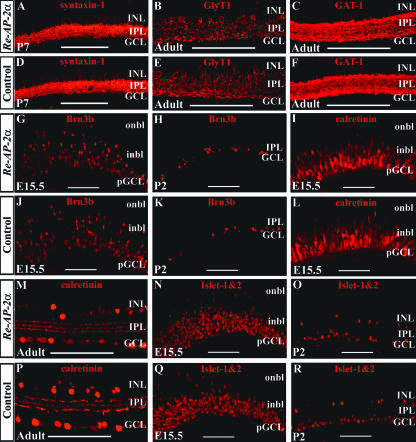
To examine Re-AP-2α mutants for possible defects in the formation of retinal cell types, Re-AP-2α and their corresponding control retinas were stained with antibodies for retinal markers (Fig. 7). To monitor amacrine cell formation in mature retinas, adult eyes were stained with antibodies for amacrine cell membrane markers. The overall appearance in the staining patterns for syntaxin-1 (Fig. 7A and D), GlyT1 (Fig. 7B and E), and GAT-1 (Fig. 7C and F) did not appear to differ in the peripheral Re-AP-2α retinas and corresponding regions in control retinas. The staining patterns of early activated inner retina markers were examined at E15.5 and a postnatal stage, to assess both early and late retinogenesis (Fig. 7G to R). The ganglion cell marker Brn3b was included to determine whether loss of AP-2α in one cell type (amacrine cells) affected other cell types, which is a common occurrence in mutant mice that are lacking one or more transcription factors required for retinal development (22). At both the embryonic and the postnatal stages examined, the staining patterns of calretinin (Fig. 7I, L, M, and P), Islet-1&2 (Fig. 7N, O, Q, and R), and Brn3b (Fig. 7G, H, J, and K) in peripheral Re-AP-2α retinas resembled those of control retinas. These results suggest that deletion of AP-2α from the retina does not visibly affect the formation of any retinal cell types.
FIG. 7.
Effect of retina-specific AP-2α deletion on the formation of different retinal cell types. Paraffin 4-μm horizontal sections that included the optic nerve were stained with either anti-syntaxin-1 (A and D), GlyT1 (B and E), GAT-1 (C and F), Brn3b (G, H, J, and K), calretinin (I, L, M, and P), or Islet-1&2 (N, O, Q, and R) antibodies. All pictures show the peripheral region, where excision of Tcfap2a occurs. Loss of AP-2α did not appear to result in a decrease in the number of cells immunoreactive for any markers tested. i/onbl, inner/outer neuroblast layer. Scale bars, 100 μm.
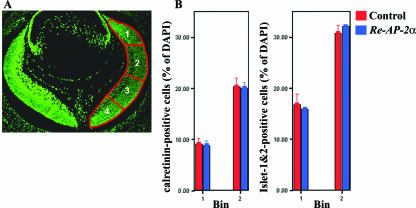
Although no overt differences between the control and mutant retinas were observed based on the staining patterns of the above-mentioned retinal markers, it was still possible that the Re-AP-2α mutants contained some subtle changes in cell numbers of the different retinal cell types. To assess whether the loss of AP-2α subtly affected the formation of different cell populations in the retina, cell counting experiments were performed using the markers calretinin and Islet-1&2. For these studies, stained sections were partitioned such that the nasal side of the retina was separated into four bins as previously described (9) (Fig. 8A), and counts were expressed as a percentage of DAPI-positive cells. Bins 1 and 2 were quantified to ensure that AP-2α was deleted from the counted regions. Sections closely preceding or following those used for counting were stained with anti-AP-2α to confirm AP-2α deletion in each mutant retina (data not shown). Counts were completed for calretinin and Islet-1&2 at E15.5 (Fig. 8B). The number of calretinin- and Islet-1&2-positive cells was not found to be altered in Re-AP-2α mutant retinas compared to control retinas.
FIG. 8.
The number of calretinin- and Islet-1&2-positive cells is not altered in Re-AP-2α mutant retinas compared to control retinas. (A) An E15.5 horizontal section showing bins used for counting. The cells in bins 1 and 2 were quantified. (B) Quantification of cells immunoreactive for calretinin and Islet-1&2 at E15.5 in Re-AP-2α and control retinas. Counts are expressed as a percentage of DAPI-positive cells (shown as the mean ± the standard error for three retinas). The Student t test was used for statistical analysis. Loss of AP-2α did not alter the number of cells immunoreactive for calretinin or Islet-1&2 at E15.5.
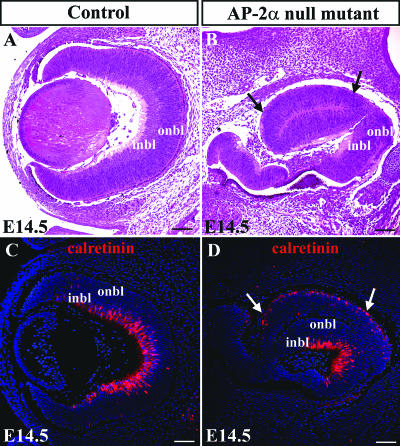
The apparent normal phenotype of the Re-AP-2α mutant retinas prompted further consideration of the non-cell-autonomous roles of AP-2α in optic cup development. This was addressed through additional examinations of the AP-2α germ line null mutants (Fig. 9). At E14.5, ocular phenotypes of the AP-2α-null mutants were variable, as previously reported (63). The phenotypes ranged from absent eyes or rudimentary eyes embedded inside the head (data not shown) to more developed eyes with multiple defects. These defects included duplication of the NR in place of RPE (Fig. 9B), which also varied in its severity. We have previously shown defects in retinal organization and lamination in newborn AP-2α-null mutants (63); however, further examination of the optic cups at an earlier stage (E14.5) revealed that lamination in the null mutants appeared to have progressed much like that of control littermates, with appropriate staining patterns for calretinin (Fig. 9C and D) and Brn3b (data not shown), markers of subsets of the two major cell populations arising at this stage, amacrine and ganglion cells. In the AP-2α-null mutants, the staining pattern of calretinin was similar to control littermates, being present in the inner retina and strongest in the central portion. Interestingly, the duplicated NR in AP-2α-null mutants expressed the inner NR marker calretinin on its outer surface, suggestive of the inverted NR reported during transdifferentiation of the RPE in the chick retina (57).
FIG. 9.
Retinal lamination in AP-2α-null mutants proceeds normally during earlier stages of development. H&E stains of representative AP-2α-null mutant (B) and control littermate (A) eyes at E14.5. (B and D) The RPE defect (conversion of RPE to a second NR) is denoted by arrows. (C and D) Staining with anticalretinin showed that retinal lamination in the null mutants appeared to resemble that of control littermates. (D) The duplicated NR in AP-2α-null mutants expressed the inner NR marker calretinin on its outer surface. Scale bars, 100 μm.
Expression of additional AP-2 family members during retinogenesis.
Given the lack of phenotypic defects in the Re-AP-2α mutant retinas, we considered the possibility that compensation by another factor was “masking” an intrinsic role for AP-2α in retinogenesis. A prime candidate transcription factor that may act redundantly with AP-2α is AP-2β, the closely related family member that is nearly identical to AP-2α in the region that spans the DNA binding and dimerization motif (41) and is the only other AP-2 family member whose transcript expression profile during retinogenesis has been directly compared to that of AP-2α and shown to be similar (3). To investigate this possibility during retinogenesis, AP-2β protein expression in the retina was examined using a mouse polyclonal anti-AP-2β antibody. Like AP-2α, AP-2β was expressed embryonically in the developing INL (Fig. 10A to D). In the developing postnatal and adult retina, AP-2β was expressed in a pattern resembling that of AP-2α, in the INL and a subset of cells in the GCL (Fig. 10E and F) and colocalized with AP-2α in a significant proportion of cells (Fig. 10I to K). This double immunostain did, however, also reveal singly stained cells expressing only AP-2α or AP-2β. Interestingly, in retinas of the Re-AP-2α mutants, regions of AP-2α deletion showed strong AP-2β expression (Fig. 10G and H).
FIG. 10.
Expression pattern of the AP-2β protein parallels that of AP-2α in the developing mouse retina. (A) At E13.5, AP-2β is expressed in the central retina, in cells of the inner neuroblast layer (or presumptive INL; arrows). (B to D) As embryogenesis progresses, AP-2β expression exhibits a central to peripheral expansion pattern in the developing INL. (E and F) By P2 and continuing throughout adulthood, AP-2β is expressed in the INL (arrows), as well as a subset of cells in the GCL (arrowheads). (G and H) Re-AP-2α mutant retinas immunostained for AP-2α (Mutant-α) or AP-2β (Mutant-β) illustrate that regions with AP-2α deletion (outlined in white) show strong expression of AP-2β. (I to K) AP-2β (red) colocalizes extensively with AP-2α (green) during embryonic (I) and postnatal (J) retinal development and in the adult retina (K). i/onbl, inner/outer neuroblast layer. Scale bars, 100 μm.
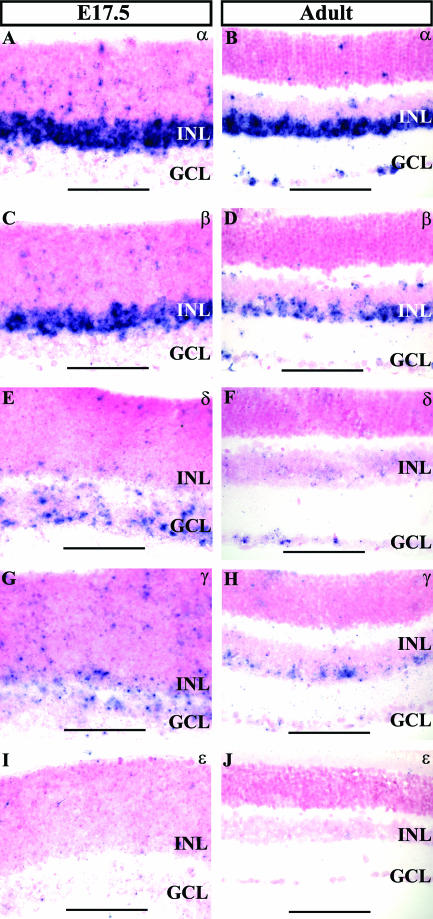
To investigate potential retinogenic roles for other AP-2 family members, we used ISH to compare the expression patterns of transcripts encoding each of the AP-2 proteins (AP-2α, AP-2β, AP-2δ, AP-2γ, and AP-2ɛ) in the embryonic and adult retina (Fig. 11). At E17.5, transcripts of both AP-2α and AP-2β were strongly expressed in the developing INL (Fig. 11A and C), in agreement with the patterns described by immunostaining. At this stage AP-2δ transcripts were present in the retina, with the majority confined to the developing GCL (Fig. 11E). AP-2γ mRNA was also observed at E17.5 in the developing INL and GCL (Fig. 11G). At no stage was a retinal signal for AP-2ɛ detected (Fig. 11I and J). In the adult retina, signals for AP-2α, AP-2β, AP-2δ, and AP-2γ were detected. AP-2α and AP-2β were expressed in the INL and GCL, as expected based on immunolocalization (Fig. 11B and D). AP-2δ transcripts remained predominantly in the GCL, as seen during development (Fig. 11F), while AP-2γ signal was present in the INL and GCL (Fig. 11H).
FIG. 11.
ISH analysis of all known AP-2 family members in the retina. (A to D) Transcripts of AP-2α and AP-2β are expressed in the developing INL at E17.5 and in the INL and GCL in the adult, in agreement with immunolocalization patterns. (E and F) AP-2δ mRNA predominates in the developing and mature GCL. (G and H) In the embryonic and adult retina, AP-2γ transcripts are expressed in the INL and GCL. (I and J) Signal for AP-2ɛ was not detected in the retina.
DISCUSSION
The combinations of transcription factors expressed by developing retinal cells are proving to be core regulators of retinogenesis. Defining the transcriptional code required for successful formation of each cell type is further complicated by the fact that these transcription factors can perform multiple functions depending on the stage of retinogenesis. Also, they may act redundantly with other related factors, raising the possibility of functional compensation and adding complexity to loss-of-function studies. We have shown here that transcription factor AP-2α exhibits a strong and distinct retinal expression pattern in postmitotic amacrine cells during retinogenesis and also in amacrine cells in the mature retina. We report successful retina-specific deletion of AP-2α but did not detect any defects in the Re-AP-2α mutant retinas, for which we will provide possible explanations below.
Expression of AP-2α in postmitotic amacrine cells.
We have previously detected AP-2α expression in the mouse retina (63) but did not conduct a detailed spatial and temporal analysis. In the present study we have shown that in the embryonic retina, AP-2α was expressed in the presumptive INL, beginning in the central retina at E13.5 and spreading to the peripheral retina in the same spatial gradient that has been described for retinal neurogenesis (Fig. 1). This central-to-peripheral expansion is a pattern commonly reported for factors involved in the genesis of one or a more limited number of cell types. For example, initial expression of Brn3b, the POU domain transcription factor required for retinal ganglion cell differentiation and survival, spreads from the central to the peripheral retina in postmitotic ganglion cells (20, 46). In the mouse, most amacrine cells are “born” (i.e., complete their final mitosis) between E12 and P1 (69); however, it is important to note that considerable lag times can exist between “cell birth” and perceptible differentiation (69). Given the time that AP-2α expression was first detected and the fact that it was expressed in postmitotic cells predominantly in the inner retina, it is likely not present in unbiased or uncommitted cells that have yet to receive intrinsic or extrinsic signals promoting amacrine cell specification. Rather, the expression pattern of AP-2α suggests that it is expressed in cells that are already committed to an amacrine cell fate and are initiating differentiation as they reach the emerging INL, where they will develop into mature amacrine cells. We have shown that AP-2α is expressed in the two major amacrine cell populations, glycinergic and GABAergic cells, as well as in cholinergic (Islet-1-positive) and calretinin-positive amacrine cells (Fig. 3). We also determined that the AP-2α-positive cells in the GCL are displaced amacrine cells and not ganglion cells (Fig. 4). These results show that AP-2α is expressed in a wide range of known amacrine cell types and may also be expressed in one or more of the “unidentified” amacrine cell classes. We did not observe a situation in which every cell positive for a given amacrine cell marker was also expressing AP-2α. Although this lack of total colocalization indicates that AP-2α is not expressed in every single amacrine cell, it is clear that a large proportion of amacrine cells are AP-2α positive. This observation, combined with the fact that AP-2α is not expressed in any other cell type, make it a valid amacrine cell marker that retains its specificity for amacrine cells in both mammalian and avian retinas (3).
A study in the developing chick retina reported an AP-2α transcript distribution that parallels our findings, with initial central-to-peripheral expansion and continued expression in amacrine cells of the INL and GCL, confirmed by colocalization of the protein with syntaxin (3). Given the developmental stage at which AP-2α expression is activated, it is a useful marker that detects early amacrine cells before they express synaptic markers such as GlyT1 but is also maintained in terminally differentiating amacrine cells and throughout adulthood. While some of the transcription factors that act earlier in the amacrine cell genetic cascade (at the level of cell fate determination) are becoming apparent, those that control postmitotic amacrine cell development are largely unknown. The bHLH factors Math3 and NeuroD act redundantly to influence amacrine cell fate specification (27), and the forkhead/winged helix transcription factor Foxn4 also promotes an amacrine cell fate, acting upstream of Math3 and NeuroD, as demonstrated by its ability to regulate their expression (31). These and other determination factors are often expressed initially in proliferating cells and may (as in the case of Foxn4) be confined exclusively to dividing progenitors. To our knowledge, just two other transcription factors in addition to AP-2α, Runx1 (54), and Barhl2 (39), have been reported in amacrine cells only after they become postmitotic. Determining how these genes interact in postmitotic amacrine cells will provide information about their differentiation and about the specification and maturation of the many amacrine cell subtypes.
Retina-specific KO of AP-2α results in a normal retina phenotype.
Previous examinations of AP-2α-null mice in our laboratory prompted us to create and analyze conditional KOs with AP-2α deleted only from the developing retina. In the AP-2α-null mutants, we observed several defects in the developing optic cup (63). These included abnormal development of the inner retina, which at birth appeared as a disorganized layer of cells lacking a distinct IPL or GCL (accompanied by absent Brn3b staining). Also, although AP-2α is not expressed in the RPE, this epithelial layer was absent on the dorsal aspect of the optic cup and replaced by a cell layer similar to the inner NR, a finding indicative of improper specification of the RPE (63). Given the severe ocular and craniofacial anomalies in these mice, it was not clear whether the optic cup defects were caused by the intrinsic loss of AP-2α from the developing retina or whether they were “indirectly” generated due to deletion of AP-2α from other tissues within or surrounding the eye. We addressed this question through examination of the retina-specific KOs. As we have shown, retina-specific deletion of AP-2α did not give rise to any of the optic cup abnormalities seen in the AP-2α-null mutants. These findings suggest that the RPE and inner retina defects of the null mutants were caused by the absence of AP-2α in other tissues and that AP-2α has non-cell-autonomous roles in optic cup development. This hypothesis is further supported by the fact that retinal lamination and patterning in the null mutant appeared to proceed normally during earlier stages of development and only during later stages was the organization in retinal lamination disrupted. Given that the ordered formation of the retina and its corresponding visual centers in the brain are partly dependent on the appropriate retina-to-brain neural connections, the loss of retinal lamination in the newborn null mutants may reflect a disruption in these connections. Finally, the fact that the RPE defect in the null mutants can vary in its severity provides further evidence that disruptions in tissue-tissue interactions is a likely cause of the defects rather than intrinsic loss of AP-2α expression.
Our thorough examination of the Re-AP-2α mutants revealed that conditional deletion of AP-2α in the mouse retina caused no detectable NR abnormalities. The Re-AP-2α mutants were indistinguishable from their control littermates at the level of overall retinal morphology (Fig. 6), staining patterns for retinal markers (Fig. 7), and counts of cells immunoreactive for calretinin or Islet-1&2 (Fig. 8). Given these results, it was necessary to consider the effectiveness of the Cre-mediated Tcfap2a deletion, including the timing and pattern of Cre recombinase activity. We confirmed by PCR that excision of the Tcfap2a allele was specific to the retina (Fig. 5B) and used a Cre indicator strain for verification of Cre activity (Fig. 5C to E). As anticipated based on the previously reported expression pattern of the Pax6 α enhancer (2, 30, 34), Cre recombinase was active in the peripheral aspects of the nasal and temporal NR, whereas the central (i.e., around optic nerve head) and dorsal NR lacked Cre activity. We consistently observed by immunofluorescence with an anti-AP-2α antibody that AP-2α was efficiently deleted in the Cre-positive areas. The epitope recognized by this antibody corresponds to the genomic region across the exon 3/4 boundary (T. Williams, unpublished data), and the floxed (Tcfap2alox) allele results in removal of a segment essential for DNA binding and dimerization across exons 5 and 6 (6). Thus, although elimination of the binding and/or dimerization domain in all likelihood renders the protein nonfunctional, it can be presumed that even if a truncated protein product was stable enough to remain, the antibody used to confirm AP-2α deletion would have detected it. Another issue to consider was the timing of Tcfap2a excision. The Pax6 α enhancer activates Cre expression at E10.5 (34). As shown in Fig. 1, the AP-2α protein was first detected at E13.5, after which the number of AP-2α-positive cells markedly increased and spread to the periphery. Using the Cre indicator strain, we showed that Cre-mediated excision had occurred where expected by E13.5, on the critical threshold of AP-2α expansion. By E15.5 it was clear that cells in the peripheral NR were AP-2α-negative (Fig. 5F), and would therefore continue to give rise to AP-2α-negative daughter cells. This was demonstrated in P2 α-Cre+/−/ROSA26+/− mice (Fig. 5E), in which the cells populating the peripheral NR were lacZ-positive, indicating Cre excision. Using the anti-AP-2α antibody, we observed a consistent absence of AP-2α in the peripheral retina at all stages of retinogenesis and in the mature retina (not shown). Thus, Cre activity occurred at the appropriate time to achieve and maintain peripheral deletion of AP-2α.
The potential effects of the AP-2α-positive cells remaining in the central and dorsal retina were also considered. One might argue that the remaining AP-2α-positive cells had influenced the AP-2α-negative cells in a non-cell-autonomous fashion, resulting in the formation of a normal retina in the conditional KOs. It is important to note, however, that while some intermingling of RPCs has been shown to occur during proliferation in the newly forming optic vesicle and optic cup (until ∼E10.5), cells arising after this stage remain within or very close to their clonal column of origin and migrate radially rather than undergoing extensive tangential dispersion (50, 51). As a result, progenitors are not progressively displaced tangentially as the retina expands. Although tangential migration of ganglion, horizontal, cone, and amacrine cells has been shown to occur, it does so over very short distances and is associated with terminal differentiation and formation of retinal mosaics (48, 49). AP-2α-positive cells were first detected days after intermingling of progenitors has ceased. Also, the migration of AP-2α-positive cells from the central to peripheral retina was never observed. Together, these data provide evidence that the formation of a normal retina in the AP-2α conditional KOs was not due to a rescue of the AP-2α-negative region by the AP-2α-positive region. Thus, our examinations of the Re-AP-2α mutant retinas suggest that AP-2α is not required cell autonomously for normal retinal development. It is also improbable that the small regions with AP-2α-positive cells could have rescued the phenotype of the whole retina. The Pax6, Shh, and Rb genes have been conditionally deleted from the developing retina via the same strain of α-Cre mice used in our study (12, 34, 61). In all cases, mutant phenotypes were observed in the peripheral retina, demonstrating successful use of this system for retinal-specific gene deletion, including the inability of any remaining “wild-type” cells in the central retina to rescue “deleted” cells that have undergone Cre excision. A more plausible explanation for the lack of retinal defects in the Re-AP-2α mutants is that another regulator is acting redundantly with AP-2α during retinogenesis and is therefore playing a compensatory role. One such candidate is AP-2β.
Possible redundancy between AP-2α and other family members in the developing retina.
Functional compensation among family members has been demonstrated for several transcription factors involved in retinogenesis, including the Brn3 family (46, 60), the Dlx homeobox genes (14), and the Hes family of bHLH transcriptional repressors (22, 28). AP-2β was the second member of the AP-2 family to be cloned and characterized (41). Comparisons of the Tcfap2a and Tcfap2b intron-exon structure and protein sequences reveal a high degree of conservation, suggesting that these two genes arose from duplication of a common ancestral gene (41-43). In the region that spans the DNA binding and dimerization motif, AP-2α and AP-2β are 92% identical at the amino acid level (41), and their expression patterns in the early embryo (E8 to E10) are nearly identical (43). These two family members have been shown to bind to each other in vitro, bind to identical sites on a given promoter, and regulate transcription of the same genes (5, 41, 73). In addition, AP-2α and AP-2β can bind to a promoter as heterodimers, as reported for the proto-oncogene c-erbB-2 promoter (5). In the chick, Bisgrove and Godbout (3) have shown by ISH that AP-2α and AP-2β were both expressed in the inner INL of the developing retina (where developing amacrine cells reside) and that the expression pattern of AP-2β was slightly more broad, also occurring in the outer INL where horizontal cells are located. We have shown that in the developing and adult mammalian retina, the pattern of AP-2β protein expression was very similar to that of AP-2α (Fig. 10A to F), with AP-2β colocalizing with AP-2α in a number of cells (Fig. 10I to K). These findings show that both family members are indeed coexpressed in a population of amacrine cells. Singly stained cells expressing either AP-2α alone or AP-2β alone were also detected, indicating that they can be independently expressed in the developing and mature retina. In addition, areas in the Re-AP-2α mutant retinas lacking AP-2α still exhibited strong AP-2β expression in the developing inner INL (Fig. 10G to H). These results indicate that both AP-2α and AP-2β are expressed in amacrine cells during retinogenesis and, given their high homology, it can be surmised that they may have functionally redundant roles in the developing retina. We have also shown that two additional AP-2 family members, AP-2δ and AP-2γ, are expressed in the developing and adult eye (Fig. 11). These data concur with previous reports in which AP-2δ mRNA was shown to be expressed in the inner mouse retina at E13.5 (70) and AP-2γ transcripts were detected in the INL and GCL of the adult mouse retina (44). Given that AP-2γ appears to be expressed in a pattern resembling that of AP-2α and AP-2β, it is possible that all three family members share similar or redundant roles during retinogenesis. The more restricted expression pattern of AP-2δ (which we detected primarily in the GCL) suggests that it is a less likely “compensatory factor” in the Re-AP-2α mutants. In subsequent investigations, it will be interesting to further document the overlapping and distinct expression patterns of AP-2 family members during retinal development.
In summary, we have shown that AP-2α was expressed in amacrine cell populations in the developing and mature retina and in both postmitotic amacrine cells during retinogenesis and differentiated amacrine cells in the INL and GCL of the adult retina. Conditional deletion of AP-2α caused no overt amacrine cell defects. Comparison of these phenotypes with that of the AP-2α-null mutants suggests that AP-2α has non-cell-autonomous roles in optic cup development. Finally, given that multiple AP-2 proteins are expressed in the developing retina and may have redundant roles, future studies that target multiple AP-2 proteins in the retina should help to uncover any intrinsic role(s) of this transcription factor family in retinogenesis.
Acknowledgments
This study was supported by National Institutes of Health grants EY11910 (J.W.-M.) and DE-12728 (T.W.) and Research to Prevent Blindness (J.W.M.).
We thank Rod Bremner for helpful advice throughout this project, and Dhruva Dwivedi for technical guidance.
Footnotes
Published ahead of print on 27 August 2007.
REFERENCES
- 1.Akagawa, K., M. Takada, H. Hayashi, and K. Uyemura. 1990. Calcium- and voltage-dependent potassium channel in the rat retinal amacrine cells identified in vitro using a cell type-specific monoclonal antibody. Brain Res. 518: 1-5. [DOI] [PubMed] [Google Scholar]
- 2.Baumer, N., T. Marquardt, A. Stoykova, R. Ashery-Padan, K. Chowdhury, and P. Gruss. 2002. Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development 129: 4535-4545. [DOI] [PubMed] [Google Scholar]
- 3.Bisgrove, D. A., and R. Godbout. 1999. Differential expression of AP-2α and AP-2β in the developing chick retina: repression of R-FABP promoter activity by AP-2. Dev. Dyn. 214: 195-206. [DOI] [PubMed] [Google Scholar]
- 4.Bisgrove, D. A., E. A. Monckton, and R. Godbout. 1997. Involvement of AP-2 in regulation of the R-FABP gene in the developing chick retina. Mol. Cell. Biol. 17: 5935-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosher, J. M., N. F. Totty, J. J. Hsuan, T. Williams, and H. C. Hurst. 1996. A family of AP-2 proteins regulates c-erbB-2 expression in mammary carcinoma. Oncogene 13: 1701-1707. [PubMed] [Google Scholar]
- 6.Brewer, S., W. Feng, J. Huang, S. Sullivan, and T. Williams. 2004. Wnt1-Cre-mediated deletion of AP-2α causes multiple neural crest-related defects. Dev. Biol. 267: 135-152. [DOI] [PubMed] [Google Scholar]
- 7.Brewer, S., X. Jiang, S. Donaldson, T. Williams, and H. M. Sucov. 2002. Requirement for AP-2α in cardiac outflow tract morphogenesis. Mech. Dev. 110: 139-149. [DOI] [PubMed] [Google Scholar]
- 8.Buettner, R., M. Moser, A. Pscherer, A. Imhof, R. Bauer, and F. Hofstaedter. 1994. Molecular cloning of a new AP-2 transcription factor, AP-2beta, and its function in cell differentiation. Verh. Dtsch. Ges. Pathol. 78: 38-42. (In German.) [PubMed] [Google Scholar]
- 9.Burmeister, M., J. Novak, M. Y. Liang, S. Basu, L. Ploder, N. L. Hawes, D. Vidgen, F. Hoover, D. Goldman, V. I. Kalnins, T. H. Roderick, B. A. Taylor, M. H. Hankin, and R. R. McInnes. 1996. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 12: 376-384. [DOI] [PubMed] [Google Scholar]
- 10.Cepko, C. L. 1999. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr. Opin. Neurobiol. 9: 37-46. [DOI] [PubMed] [Google Scholar]
- 11.Cepko, C. L., C. P. Austin, X. Yang, M. Alexiades, and D. Ezzeddine. 1996. Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA 93: 589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, D., I. Livne-bar, J. L. Vanderluit, R. S. Slack, M. Agochiya, and R. Bremner. 2004. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell 5: 539-551. [DOI] [PubMed] [Google Scholar]
- 13.Chow, R. L., and R. A. Lang. 2001. Early eye development in vertebrates. Annu. Rev. Cell Dev. Biol. 17: 255-296. [DOI] [PubMed] [Google Scholar]
- 14.de Melo, J., G. Du, M. Fonseca, L. A. Gillespie, W. J. Turk, J. L. Rubenstein, and D. D. Eisenstat. 2005. Dlx1 and Dlx2 function is necessary for terminal differentiation and survival of late-born retinal ganglion cells in the developing mouse retina. Development 132: 311-322. [DOI] [PubMed] [Google Scholar]
- 15.de Melo, J., X. Qiu, G. Du, L. Cristante, and D. D. Eisenstat. 2003. Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J. Comp. Neurol. 461: 187-204. [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi, D. J., G. F. Pontoriero, R. Ashery-Padan, S. Sullivan, T. Williams, and J. A. West-Mays. 2005. Targeted deletion of AP-2alpha leads to disruption in corneal epithelial cell integrity and defects in the corneal stroma. Investig. Ophthalmol. Vis. Sci. 46: 3623-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckert, D., S. Buhl, S. Weber, R. Jager, and H. Schorle. 2005. The AP-2 family of transcription factors. Genome Biol. 6: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng, W., and T. Williams. 2003. Cloning and characterization of the mouse AP-2 epsilon gene: a novel family member expressed in the developing olfactory bulb. Mol. Cell Neurosci. 24: 460-475. [DOI] [PubMed] [Google Scholar]
- 19.Galli-Resta, L., G. Resta, S. S. Tan, and B. E. Reese. 1997. Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. J. Neurosci. 17: 7831-7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gan, L., S. W. Wang, Z. Huang, and W. H. Klein. 1999. POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Dev. Biol. 210: 469-480. [DOI] [PubMed] [Google Scholar]
- 21.Gan, L., M. Xiang, L. Zhou, D. S. Wagner, W. H. Klein, and J. Nathans. 1996. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc. Natl. Acad. Sci. USA 93: 3920-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatakeyama, J., and R. Kageyama. 2004. Retinal cell fate determination and bHLH factors. Semin. Cell Dev. Biol. 15: 83-89. [DOI] [PubMed] [Google Scholar]
- 23.Haverkamp, S., F. Haeseleer, and A. Hendrickson. 2003. A comparison of immunocytochemical markers to identify bipolar cell types in human and monkey retina. Vis. Neurosci. 20: 589-600. [DOI] [PubMed] [Google Scholar]
- 24.Hilger-Eversheim, K., M. Moser, H. Schorle, and R. Buettner. 2000. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene 260: 1-12. [DOI] [PubMed] [Google Scholar]
- 25.Hinds, J. W., and P. L. Hinds. 1978. Early development of amacrine cells in the mouse retina: an electron microscopic, serial section analysis. J. Comp. Neurol. 179: 277-300. [DOI] [PubMed] [Google Scholar]
- 26.Hu, M., and S. S. Easter. 1999. Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Dev. Biol. 207: 309-321. [DOI] [PubMed] [Google Scholar]
- 27.Inoue, T., M. Hojo, Y. Bessho, Y. Tano, J. E. Lee, and R. Kageyama. 2002. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development 129: 831-842. [DOI] [PubMed] [Google Scholar]
- 28.Kageyama, R., J. Hatakeyama, and T. Ohtsuka. 2006. Roles of Hes bHLH factors in neural development, p. 3-22. In G. Thiel (ed.), Transcription factors in the nervous system: development, brain function, and diseases. Wiley, Hoboken, NJ.
- 29.Kageyama, R., T. Ohtsuka, J. Hatakeyama, and R. Ohsawa. 2005. Roles of bHLH genes in neural stem cell differentiation. Exp. Cell Res. 306: 343-348. [DOI] [PubMed] [Google Scholar]
- 30.Kammandel, B., K. Chowdhury, A. Stoykova, S. Aparicio, S. Brenner, and P. Gruss. 1999. Distinct cis-essential modules direct the time-space pattern of the Pax6 gene activity. Dev. Biol. 205: 79-97. [DOI] [PubMed] [Google Scholar]
- 31.Li, S., Z. Mo, X. Yang, S. M. Price, M. M. Shen, and M. Xiang. 2004. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron 43: 795-807. [DOI] [PubMed] [Google Scholar]
- 32.Livesey, F. J., and C. L. Cepko. 2001. Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2: 109-118. [DOI] [PubMed] [Google Scholar]
- 33.Marquardt, T. 2003. Transcriptional control of neuronal diversification in the retina. Prog. Retin. Eye Res. 22: 567-577. [DOI] [PubMed] [Google Scholar]
- 34.Marquardt, T., R. Ashery-Padan, N. Andrejewski, R. Scardigli, F. Guillemot, and P. Gruss. 2001. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105: 43-55. [DOI] [PubMed] [Google Scholar]
- 35.Marquardt, T., and P. Gruss. 2002. Generating neuronal diversity in the retina: one for nearly all. Trends Neurosci. 25: 32-38. [DOI] [PubMed] [Google Scholar]
- 36.Masland, R. H. 1988. Amacrine cells. Trends Neurosci. 11: 405-410. [DOI] [PubMed] [Google Scholar]
- 37.McCabe, K. L., E. C. Gunther, and T. A. Reh. 1999. The development of the pattern of retinal ganglion cells in the chick retina: mechanisms that control differentiation. Development 126: 5713-5724. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell, P. J., P. M. Timmons, J. M. Hebert, P. W. Rigby, and R. Tjian. 1991. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 5: 105-119. [DOI] [PubMed] [Google Scholar]
- 39.Mo, Z., S. Li, X. Yang, and M. Xiang. 2004. Role of the Barhl2 homeobox gene in the specification of glycinergic amacrine cells. Development 131: 1607-1618. [DOI] [PubMed] [Google Scholar]
- 40.Morrow, E. M., T. Furukawa, J. E. Lee, and C. L. Cepko. 1999. NeuroD regulates multiple functions in the developing neural retina in rodent. Development 126: 23-36. [DOI] [PubMed] [Google Scholar]
- 41.Moser, M., A. Imhof, A. Pscherer, R. Bauer, W. Amselgruber, F. Sinowatz, F. Hofstadter, R. Schule, and R. Buettner. 1995. Cloning and characterization of a second AP-2 transcription factor: AP-2β. Development 121: 2779-2788. [DOI] [PubMed] [Google Scholar]
- 42.Moser, M., A. Pscherer, C. Roth, J. Becker, G. Mucher, K. Zerres, C. Dixkens, J. Weis, L. Guay-Woodford, R. Buettner, and R. Fassler. 1997. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2β. Genes Dev. 11: 1938-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moser, M., J. Ruschoff, and R. Buettner. 1997. Comparative analysis of AP-2α and AP-2β gene expression during murine embryogenesis. Dev. Dyn. 208: 115-124. [DOI] [PubMed] [Google Scholar]
- 44.Oulad-Abdelghani, M., P. Bouillet, C. Chazaud, P. Dolle, and P. Chambon. 1996. AP-2.2: a novel AP-2-related transcription factor induced by retinoic acid during differentiation of P19 embryonal carcinoma cells. Exp. Cell Res. 225: 338-347. [DOI] [PubMed] [Google Scholar]
- 45.Pak, W., R. Hindges, Y. S. Lim, S. L. Pfaff, and D. D. O'Leary. 2004. Magnitude of binocular vision controlled by islet-2 repression of a genetic program that specifies laterality of retinal axon pathfinding. Cell 119: 567-578. [DOI] [PubMed] [Google Scholar]
- 46.Pan, L., Z. Yang, L. Feng, and L. Gan. 2005. Functional equivalence of Brn3 POU-domain transcription factors in mouse retinal neurogenesis. Development 132: 703-712. [DOI] [PubMed] [Google Scholar]
- 47.Rapaport, D. H., L. L. Wong, E. D. Wood, D. Yasumura, and M. M. LaVail. 2004. Timing and topography of cell genesis in the rat retina. J. Comp. Neurol. 474: 304-324. [DOI] [PubMed] [Google Scholar]
- 48.Reese, B. E., and L. Galli-Resta. 2002. The role of tangential dispersion in retinal mosaic formation. Prog. Retin. Eye Res. 21: 153-168. [DOI] [PubMed] [Google Scholar]
- 49.Reese, B. E., A. R. Harvey, and S. S. Tan. 1995. Radial and tangential dispersion patterns in the mouse retina are cell-class specific. Proc. Natl. Acad. Sci. USA 92: 2494-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reese, B. E., B. D. Necessary, P. P. Tam, B. Faulkner-Jones, and S. S. Tan. 1999. Clonal expansion and cell dispersion in the developing mouse retina. Eur. J. Neurosci. 11: 2965-2978. [DOI] [PubMed] [Google Scholar]
- 51.Reese, B. E., and S. S. Tan. 1998. Clonal boundary analysis in the developing retina using X-inactivation transgenic mosaic mice. Semin. Cell Dev. Biol. 9: 285-292. [DOI] [PubMed] [Google Scholar]
- 52.Schaeren-Wiemers, N., and A. Gerfin-Moser. 1993. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labeled cRNA probes. Histochemistry 100: 431-440. [DOI] [PubMed] [Google Scholar]
- 53.Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21: 70-71. [DOI] [PubMed] [Google Scholar]
- 54.Stewart, L., M. A. Potok, S. A. Camper, and S. Stifani. 2005. Runx1 expression defines a subpopulation of displaced amacrine cells in the developing mouse retina. J. Neurochem. 94: 1739-1745. [DOI] [PubMed] [Google Scholar]
- 55.Strettoi, E., and R. H. Masland. 1996. The number of unidentified amacrine cells in the mammalian retina. Proc. Natl. Acad. Sci. USA 93: 14906-14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomita, K., K. Moriyoshi, S. Nakanishi, F. Guillemot, and R. Kageyama. 2000. Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. EMBO J. 19: 5460-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsonis, P. A., and K. Del Rio-Tsonis. 2004. Lens and retina regeneration: transdifferentiation, stem cells, and clinical applications. Exp. Eye Res. 78: 161-172. [DOI] [PubMed] [Google Scholar]
- 58.Tsuchida, T., M. Ensini, S. B. Morton, M. Baldassare, T. Edlund, T. M. Jessell, and S. L. Pfaff. 1994. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell 79: 957-970. [DOI] [PubMed] [Google Scholar]
- 59.Vaney, D. I. 1990. The mosaic of amacrine cells in the mammalian retina. Prog. Ret Res. 9: 49-100. [Google Scholar]
- 60.Wang, S. W., X. Mu, W. J. Bowers, D. S. Kim, D. J. Plas, M. C. Crair, H. J. Federoff, L. Gan, and W. H. Klein. 2002. Brn3b/Brn3c double knockout mice reveal an unsuspected role for Brn3c in retinal ganglion cell axon outgrowth. Development 129: 467-477. [DOI] [PubMed] [Google Scholar]
- 61.Wang, Y., G. D. Dakubo, S. Thurig, C. J. Mazerolle, and V. A. Wallace. 2005. Retinal ganglion cell-derived sonic hedgehog locally controls proliferation and the timing of RGC development in the embryonic mouse retina. Development 132: 5103-5113. [DOI] [PubMed] [Google Scholar]
- 62.West-Mays, J. A., B. M. Coyle, J. Piatigorsky, S. Papagiotas, and D. Libby. 2002. Ectopic expression of AP-2α transcription factor in the lens disrupts fiber cell differentiation. Dev. Biol. 245: 13-27. [DOI] [PubMed] [Google Scholar]
- 63.West-Mays, J. A., J. Zhang, T. Nottoli, S. Hagopian-Donaldson, D. Libby, K. J. Strissel, and T. Williams. 1999. AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev. Biol. 206: 46-62. [DOI] [PubMed] [Google Scholar]
- 64.Williams, T., A. Admon, B. Luscher, and R. Tjian. 1988. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 2: 1557-1569. [DOI] [PubMed] [Google Scholar]
- 65.Williams, T., and R. Tjian. 1991. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 5: 670-682. [DOI] [PubMed] [Google Scholar]
- 66.Williams, T., and R. Tjian. 1991. Characterization of a dimerization motif in AP-2 and its function in heterologous DNA-binding proteins. Science 251: 1067-1071. [DOI] [PubMed] [Google Scholar]
- 67.Xiang, M., L. Zhou, J. P. Macke, T. Yoshioka, S. H. Hendry, R. L. Eddy, T. B. Shows, and J. Nathans. 1995. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J. Neurosci. 15: 4762-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiang, M., L. Zhou, Y. W. Peng, R. L. Eddy, T. B. Shows, and J. Nathans. 1993. Brn-3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron 11: 689-701. [DOI] [PubMed] [Google Scholar]
- 69.Young, R. W. 1985. Cell differentiation in the retina of the mouse. Anat. Rec. 212: 199-205. [DOI] [PubMed] [Google Scholar]
- 70.Zhao, F., T. Lufkin, and B. D. Gelb. 2003. Expression of Tfap2d, the gene encoding the transcription factor Ap-2 delta, during mouse embryogenesis. Gene Expr. Patterns 3: 213-217. [DOI] [PubMed] [Google Scholar]
- 71.Zhao, F., M. Satoda, J. D. Licht, Y. Hayashizaki, and B. D. Gelb. 2001. Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2δ, with unique DNA binding and transactivation properties. J. Biol. Chem. 276: 40755-40760. [DOI] [PubMed] [Google Scholar]
- 72.Zhou, J., and D. M. Kochhar. 2003. Regulation of AP-2 and apoptosis in developing eye in a vitamin A-deficiency model. Birth Defects Res. A Clin. Mol. Teratol. 67: 41-53. [DOI] [PubMed] [Google Scholar]
- 73.Zhu, C. H., Y. Huang, L. W. Oberley, and F. E. Domann. 2001. A family of AP-2 proteins downregulate manganese superoxide dismutase expression. J. Biol. Chem. 276: 14407-14413. [DOI] [PubMed] [Google Scholar]