Abstract
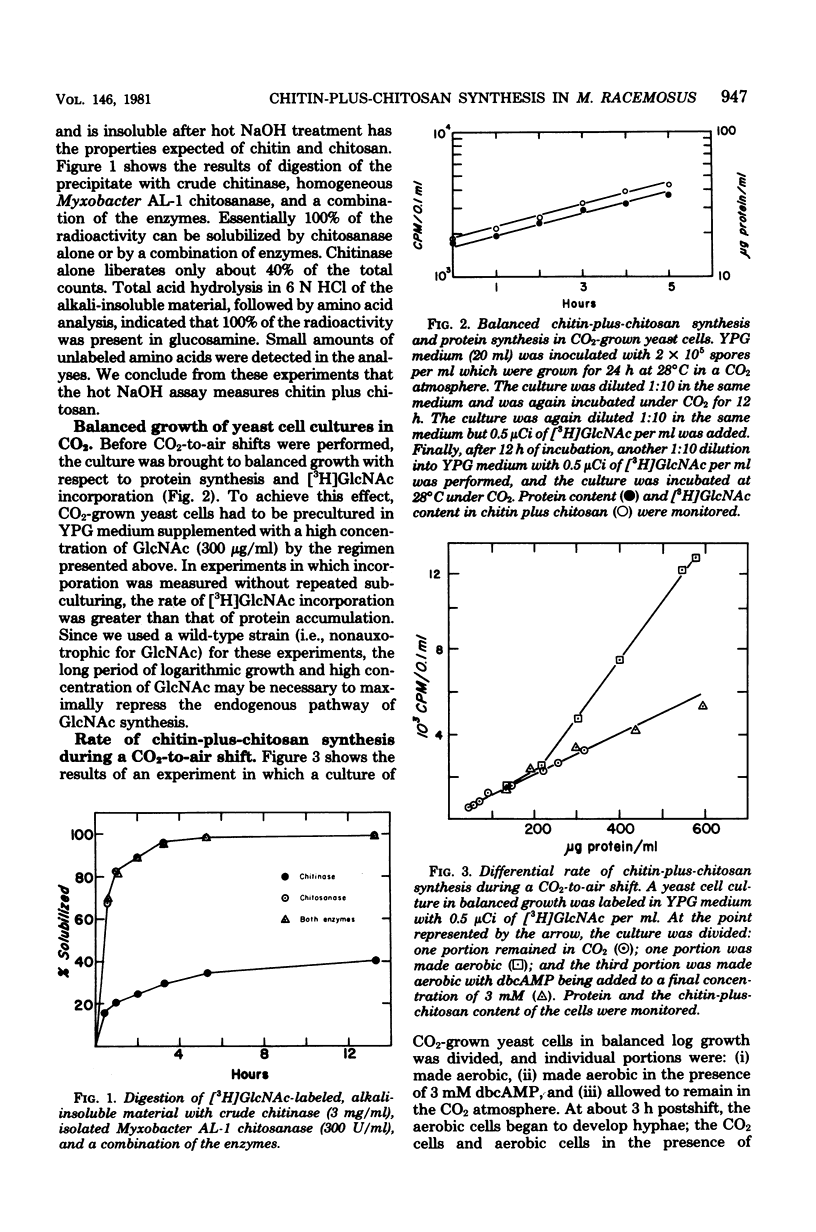
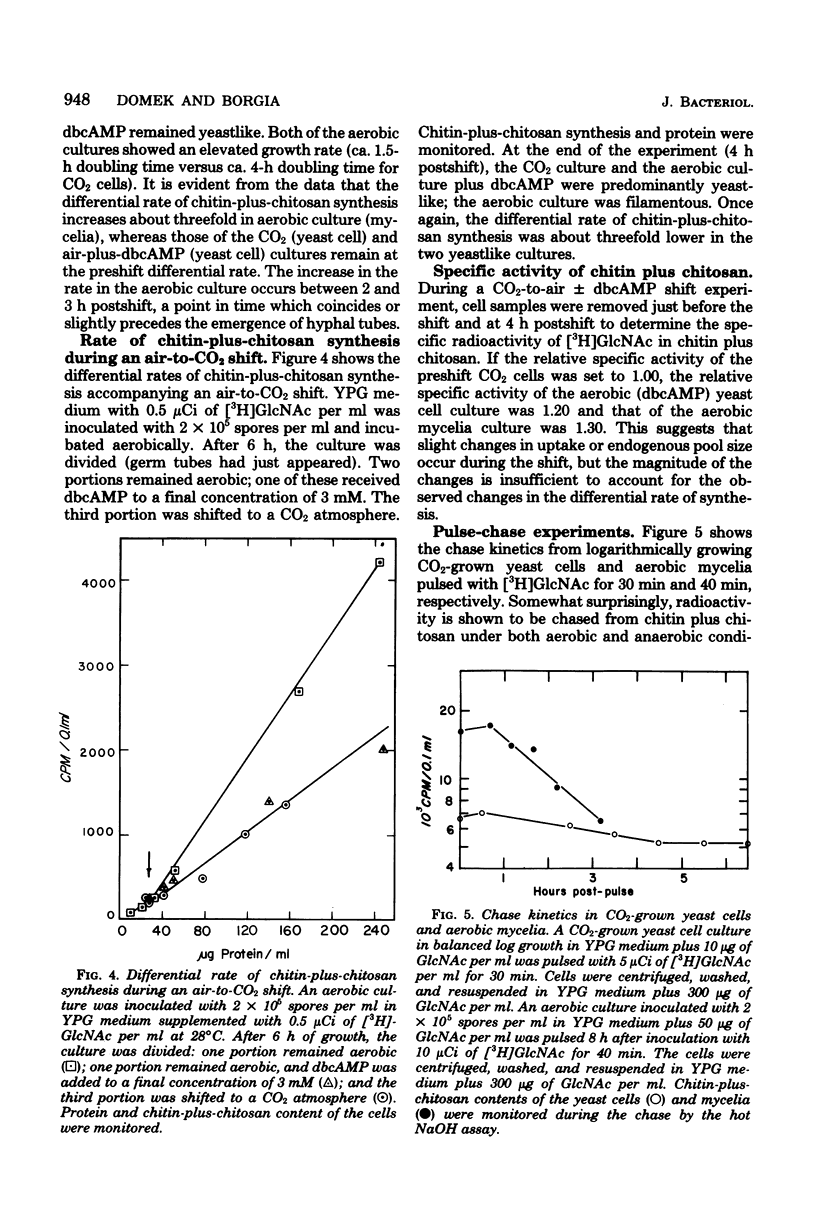
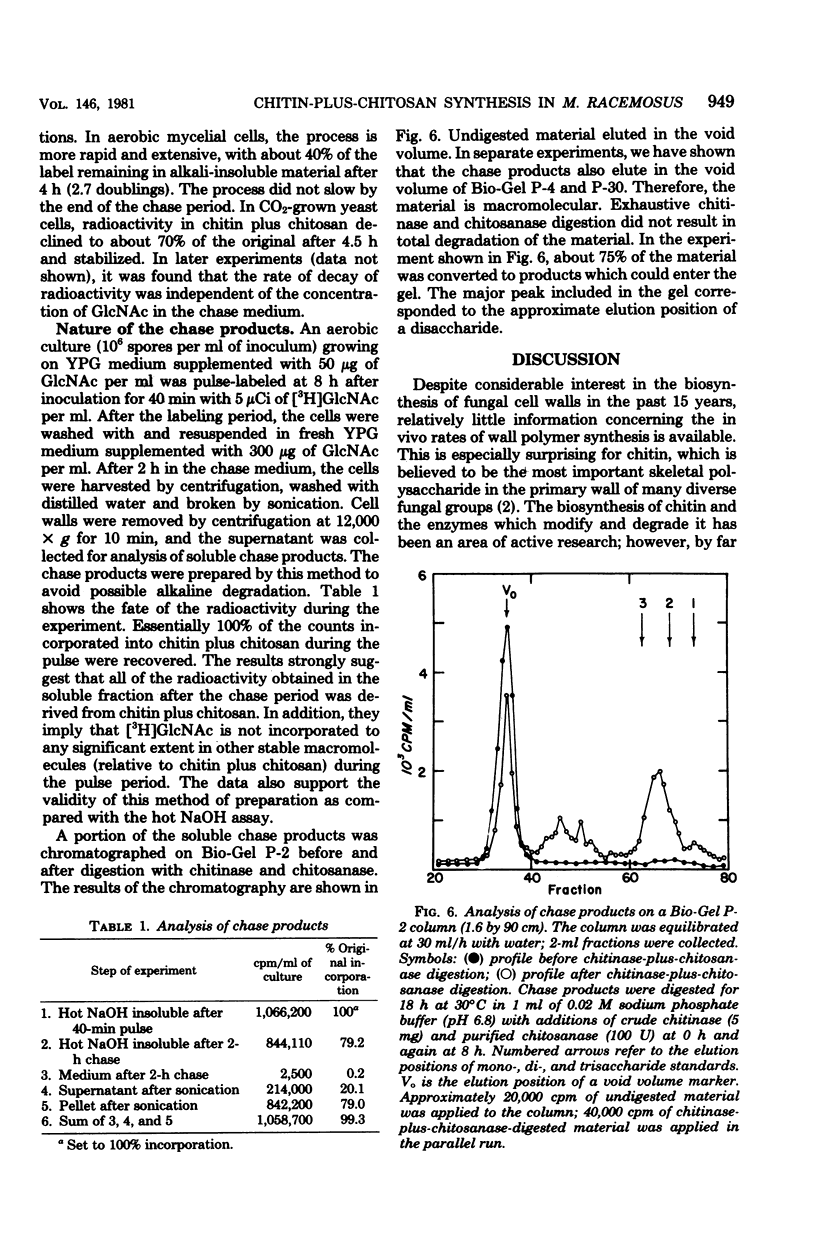
The in vivo differential rates of chitin-plus-chitosan biosynthesis in Mucor racemosus were determined under a variety of conditions, leading to yeast cell or mycelial morphology. Chitin-chitosan was determined as hot NaOH-insoluble radioactivity derived from N-acetyl-D-[1-3H]glucosamine in the medium. Control experiments demonstrated that the labeled material possessed the properties of chitin-plus-chitosan. Our results indicate that Mucor yeasts have a relatively low differential rate of chitin-plus-chitosan synthesis and that mycelial cells have a threefold-elevated differential rate. Treatment of aerobic cells with exogenous N6, O2-dibutyryl cyclic adenosine 3',5'-monophosphate, an agent which induces yeast cell morphology, also results in a lowered rate of chitin-plus-chitosan synthesis. Control experiments eliminated the possibility that the observed rate changes were due to changes in endogenous pool size, uptake of exogenous N-acetyl-p-[1-3H]glucosamine, or alterations in growth rate. Therefore, the changes are seemingly linked to morphogenesis. These results strengthen the idea that cyclic adenosine 3',5'-monophosphate plays an important role in dimorphism in Mucor. In addition, pulse-chase experiments suggest that considerable modification of newly synthesized chitin plus chitosan in both yeast cells and mycelia occurs in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Ito E. A pathway of chitosan formation in Mucor rouxii: enzymatic deacetylation of chitin. Biochem Biophys Res Commun. 1974 Feb 4;56(3):669–675. doi: 10.1016/0006-291x(74)90657-3. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Lippman E. Fungal morphogenesis: cell wall construction in Mucor rouxii. Science. 1969 Jul 18;165(3890):302–304. doi: 10.1126/science.165.3890.302. [DOI] [PubMed] [Google Scholar]
- Borgia P., Sypherd P. S. Control of beta-glucosidase synthesis in Mucor racemosus. J Bacteriol. 1977 May;130(2):812–817. doi: 10.1128/jb.130.2.812-817.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracker C. E., Ruiz-Herrera J., Bartnicki-Garcia S. Structure and transformation of chitin synthetase particles (chitosomes) during microfibril synthesis in vitro. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4570–4574. doi: 10.1073/pnas.73.12.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A., Cabib E., Bowers B. Chitin synthetase distribution on the yeast plasma membrane. Science. 1979 Jan 26;203(4378):363–365. doi: 10.1126/science.366747. [DOI] [PubMed] [Google Scholar]
- Epstein P. M., Silverman P. M. Induction of cyclic AMP phosphodiesterase in Blastocladiella emersonii and its relation to cyclic AMP metabolism. J Bacteriol. 1978 Sep;135(3):968–975. doi: 10.1128/jb.135.3.968-975.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas V. Biosynthesis of cell walls of fungi. Microbiol Rev. 1979 Jun;43(2):117–144. doi: 10.1128/mr.43.2.117-144.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges A., Wolfe R. S. Extracellular enzyme from Myxobacter AL-1 that exhibits both beta-1,4-glucanase and chitosanase activites. J Bacteriol. 1974 Nov;120(2):844–853. doi: 10.1128/jb.120.2.844-853.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsley D., Kay D. Wall structure of the Neurospora hyphal apex: immunofluorescent localization of wall surface antigens. J Gen Microbiol. 1976 Aug;96(2):233–248. doi: 10.1099/00221287-95-2-233. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen A. D., Sypherd P. S. Cyclic adenosine 3',5'-monophosphate and morphogenesis in Mucor racemosus. J Bacteriol. 1974 Feb;117(2):432–438. doi: 10.1128/jb.117.2.432-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M. L. Cyclic AMP and the plasma membrane potential in Neurospora crassa. J Biol Chem. 1977 Oct 25;252(20):7146–7150. [PubMed] [Google Scholar]
- Paveto C., Epstein A., Passeron S. Studies on cyclic adenosine 3' ,5'-monophosphate levels, Adenylate cyclase and phosphodiesterase activities in the dimorphic fungus Mucor rouxii. Arch Biochem Biophys. 1975 Aug;169(2):449–457. doi: 10.1016/0003-9861(75)90187-3. [DOI] [PubMed] [Google Scholar]
- Polacheck I., Rosenberger R. F. Aspergillus nidulans mutant lacking alpha-(1,3)-glucan, melanin, and cleistothecia. J Bacteriol. 1977 Nov;132(2):650–656. doi: 10.1128/jb.132.2.650-656.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G., Pall M. L. Properties of two cyclic nucleotide-deficient mutants of Neurospora crassa. J Bacteriol. 1979 Mar;137(3):1140–1144. doi: 10.1128/jb.137.3.1140-1144.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi H. F., Flawia M. M., Tellez-Inon M. T., Torres H. N. Control of Neurospora crassa morphology by cyclic adenosine 3', 5'-monophosphate and dibutyryl cyclic adenosine 3', 5'-monophosphate. J Bacteriol. 1976 Apr;126(1):91–99. doi: 10.1128/jb.126.1.91-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevillyan J. M., Pall M. L. Control of cyclic adenosine 3',5'-monophosphate levels by depolarizing agents in fungi. J Bacteriol. 1979 May;138(2):397–403. doi: 10.1128/jb.138.2.397-403.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J. C., Malhotra S. K. The significance of cAMP induced alterations in the cellular structure of Phycomyces. Can J Microbiol. 1977 Apr;23(4):378–388. doi: 10.1139/m77-056. [DOI] [PubMed] [Google Scholar]
- Uno I., Ishikawa T. Purification and identification of the fruiting-inducing substances in Coprinus macrorhizus. J Bacteriol. 1973 Mar;113(3):1240–1248. doi: 10.1128/jb.113.3.1240-1248.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld B. J. The effect of glucose and manganese on adenosine-3',5'-monophosphate levels during growth and differentiation of Aspergillus nidulans. Arch Microbiol. 1976 May 3;108(1):41–44. doi: 10.1007/BF00425091. [DOI] [PubMed] [Google Scholar]


