Abstract
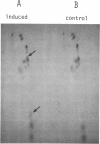
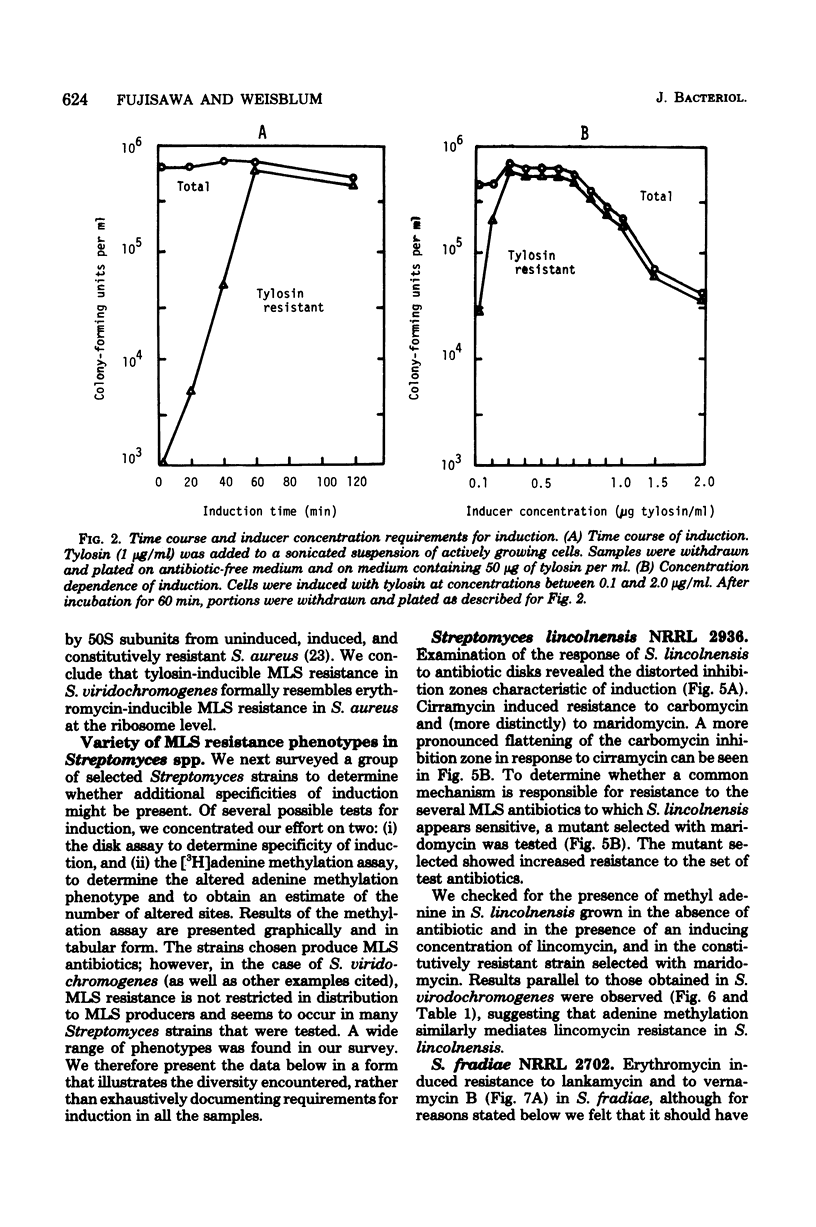
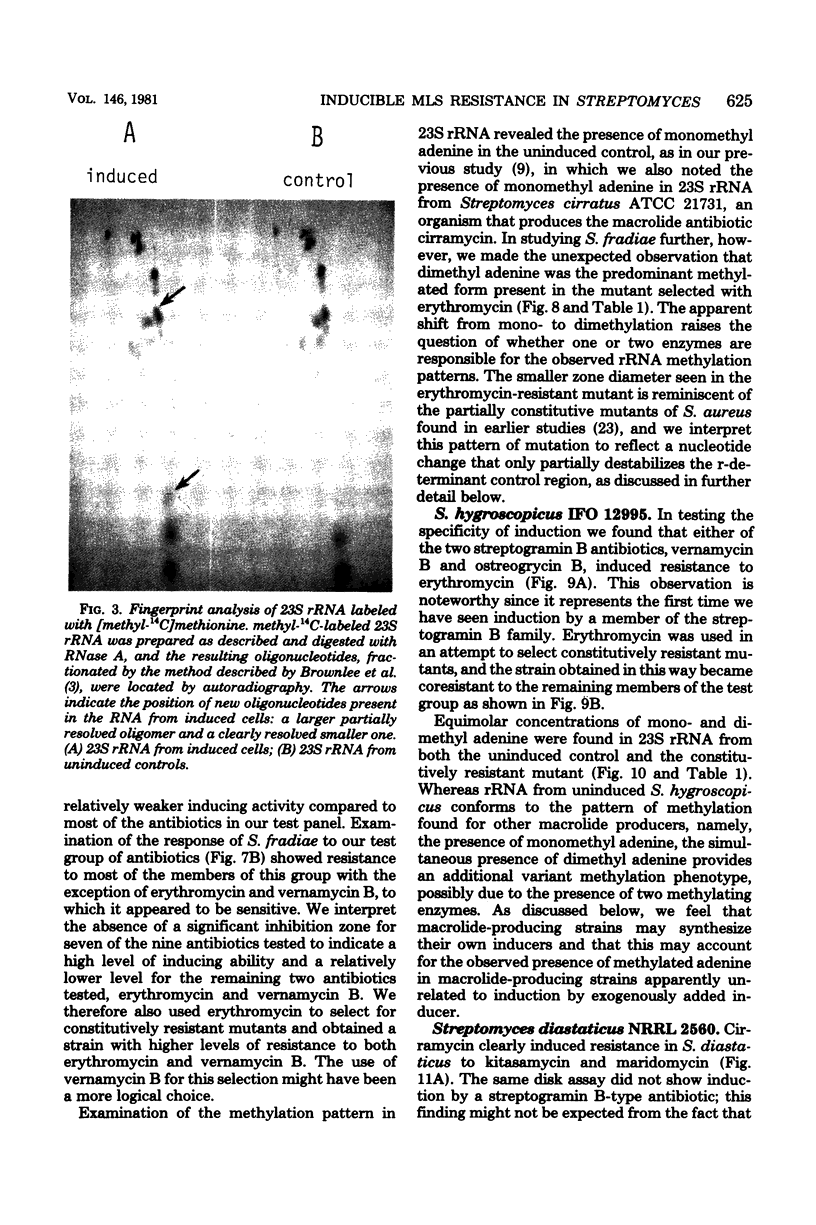
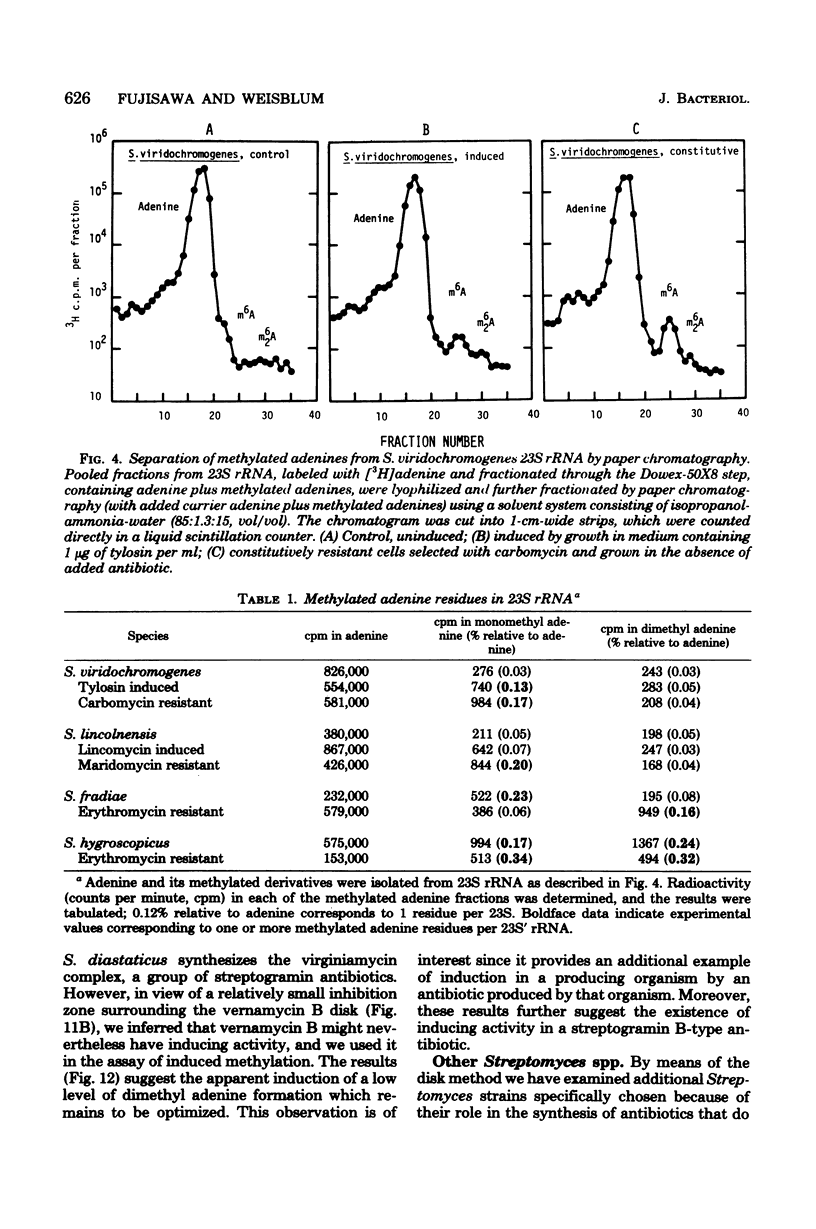
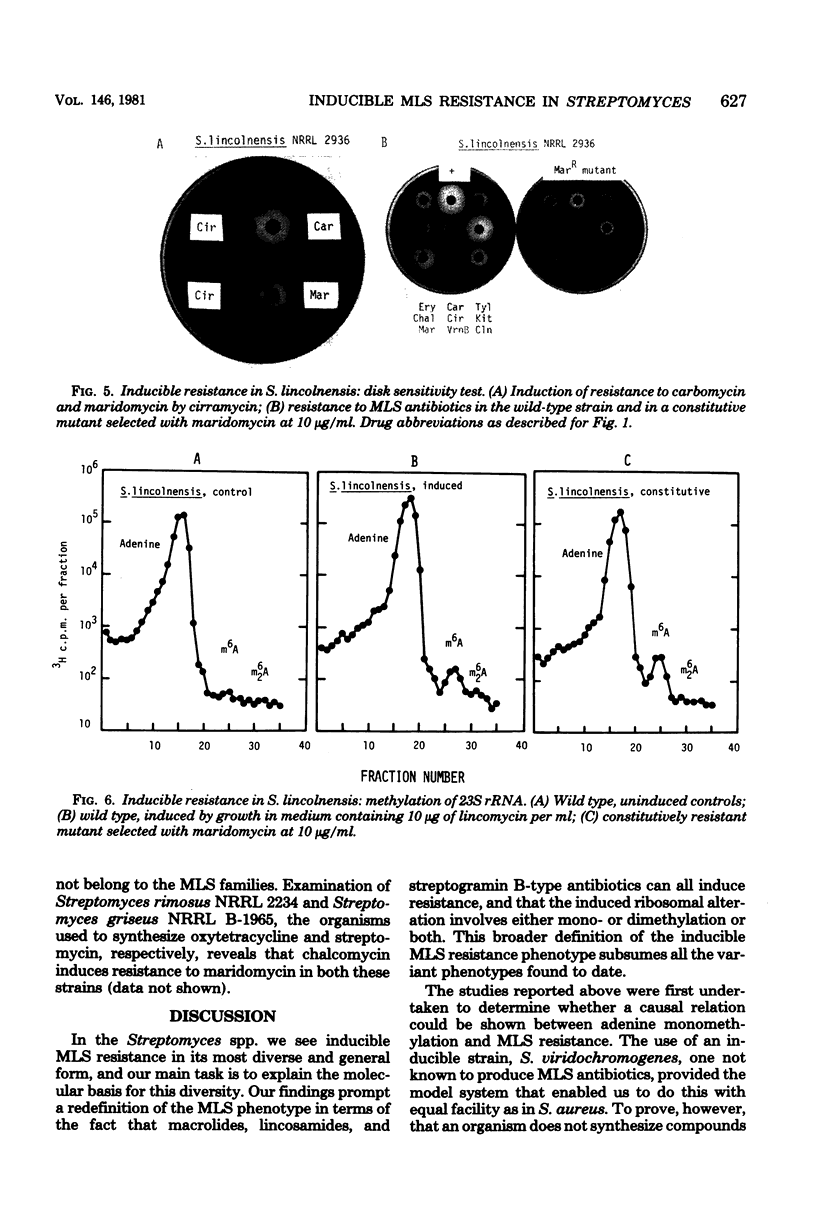
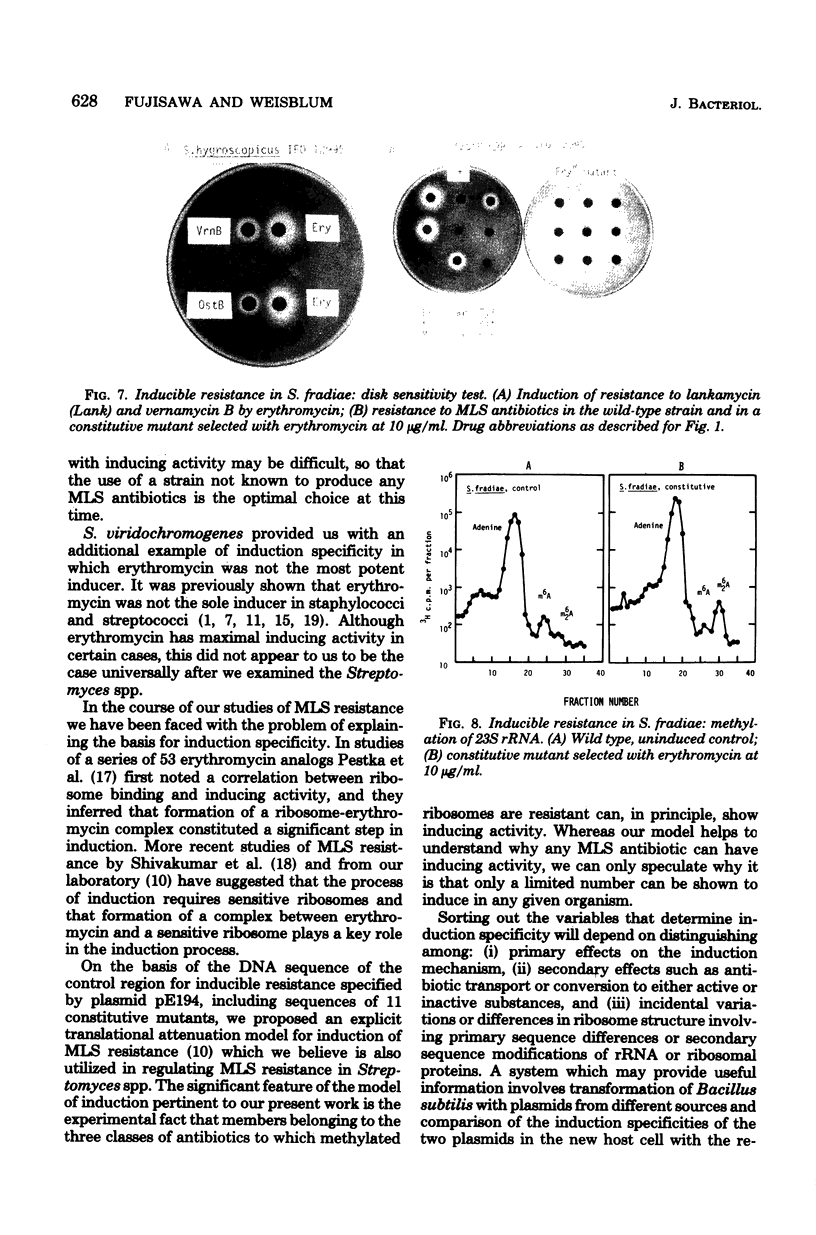
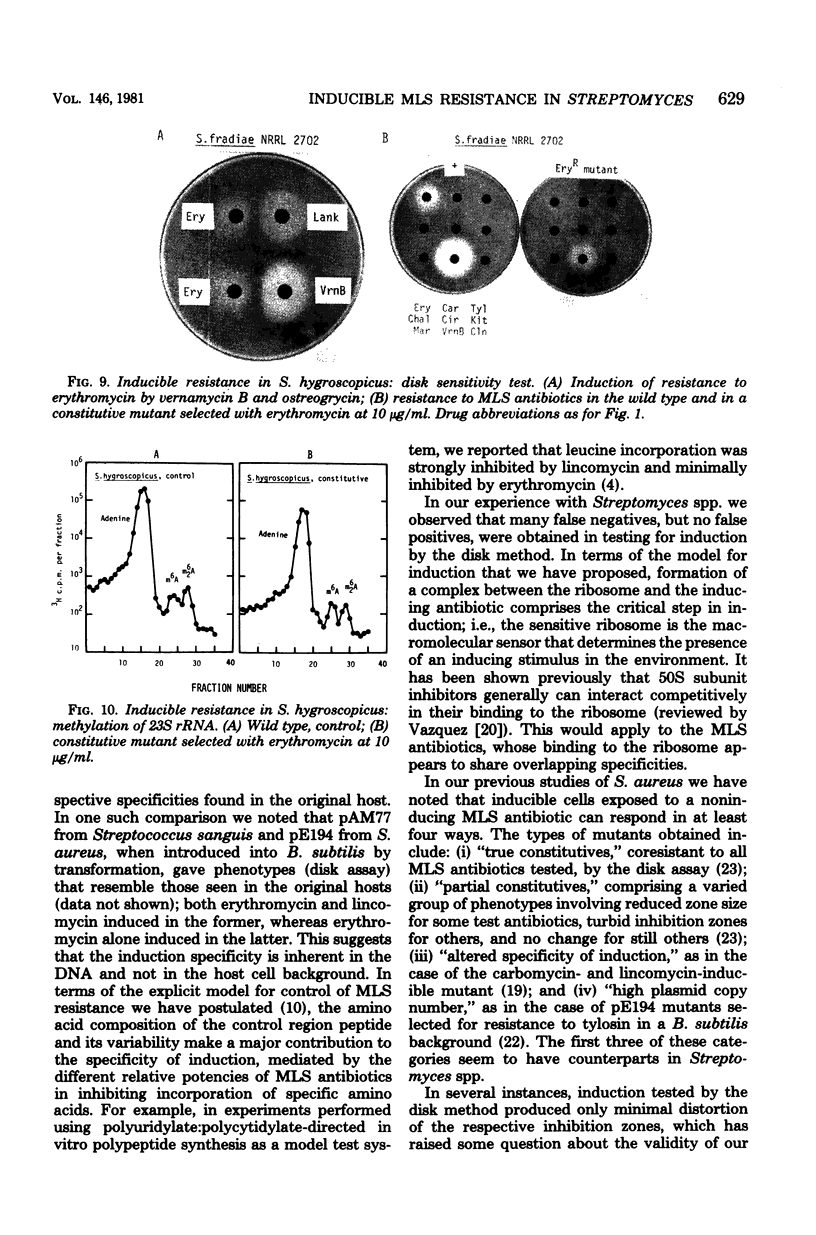
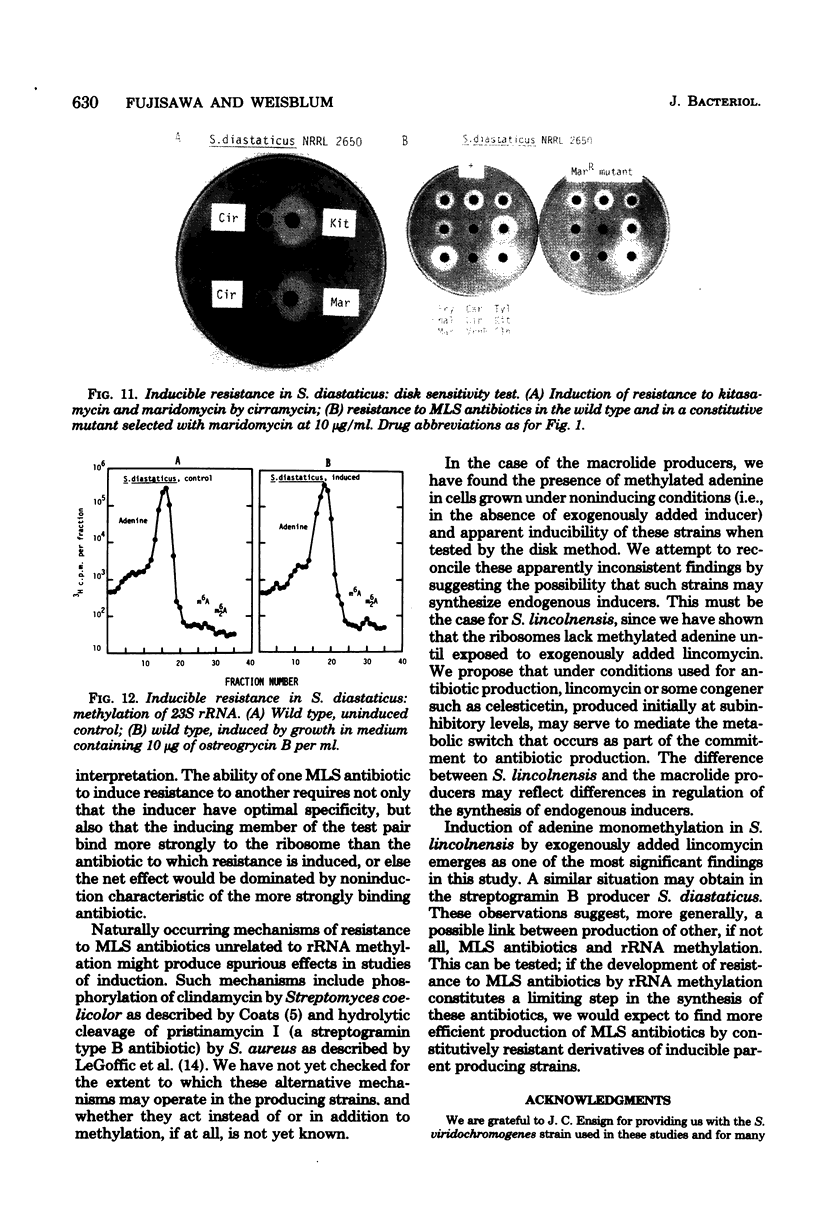
Inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics in Streptomyces spp. comprises a family of diverse phenotypes in which characteristic subsets of the macrolide-lincosamide-streptogramin antibiotics induce resistance mediated by mono- or dimethylation of adenine, or both, in 23S ribosomal ribonucleic acid. In these studies, diverse patterns of induction specificity in Streptomyces and associated ribosomal ribonucleic acid changes are described. In Streptomyces fradiae NRRL 2702 erythromycin induced resistance to vernamycin B, whereas in Streptomyces hygroscopicus IFO 12995, the reverse was found: vernamycin B induced resistance to erythromycin. In a Streptomyces viridochromogenes (NRRL 2860) model system studied in detail, tylosin induced resistance to erythromycin associated with N6-monomethylation of 23S ribosomal ribonucleic acid, whereas in Staphylococcus aureus, erythromycin induced resistance to tylosin mediated by N6-dimethylation of adenine. Inducible macrolide-lincosamide-streptogramin resistance was found in S. fradiae NRRL 2702 and S. hygroscopicus IFO 12995, which synthesize the macrolides tylosin and maridomycin, respectively, as well as in the lincosamide producer Streptomyces lincolnensis NRRL 2936 and the streptogramin type B producer Streptomyces diastaticus NRRL 2560. A wide range of different macrolides including chalcomycin, tylosin, and cirramycin induced resistance when tested in an appropriate system. Lincomycin was active as inducer in S. lincolnensis, the organism by which it is produced, and streptogramin type B antibiotics induced resistance in S. fradiae, S. hygroscopicus, and the streptogramin type B producer S. diastaticus. Patterns of adenine methylation found included (i) lincomycin-induced monomethylation in S. lincolnensis (and constitutive monomethylation in a mutant selected with maridomycin), (ii) concurrent equimolar levels of adenine mono- plus dimethylation in S. hygroscopicus, (iii) monomethylation in S. fradiae (and dimethylation in a mutant selected with erythromycin), and (iv) adenine dimethylation in S. diastaticus induced by ostreogrycin B.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E. Macrolide resistance in Staphylococcus aureus: inducers of macrolide resistance. Antimicrob Agents Chemother. 1977 Apr;11(4):669–674. doi: 10.1128/aac.11.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz R. H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. J Gen Microbiol. 1978 Jul;107(1):93–102. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. The sequence of 5 s ribosomal ribonucleic acid. J Mol Biol. 1968 Jun 28;34(3):379–412. doi: 10.1016/0022-2836(68)90168-x. [DOI] [PubMed] [Google Scholar]
- Chang F. N., Weisblum B. The specificity of lincomycin binding to ribosomes. Biochemistry. 1967 Mar;6(3):836–843. doi: 10.1021/bi00855a025. [DOI] [PubMed] [Google Scholar]
- Coats J. H. Clindamycin hosphotransferase. Methods Enzymol. 1975;43:755–759. doi: 10.1016/0076-6879(75)43142-1. [DOI] [PubMed] [Google Scholar]
- Dixon J. M., Lipinski A. E. Infections with beta-Hemolytic Streptococcus resistant to lincomycin and erythromycin and observations on zonal-pattern resistance to lincomycin. J Infect Dis. 1974 Oct;130(4):351–356. doi: 10.1093/infdis/130.4.351. [DOI] [PubMed] [Google Scholar]
- Dixon J. M., Lipinski A. E. Resistance of group A beta-hemolytic streptococci to lincomycin and erythromycin. Antimicrob Agents Chemother. 1972 Apr;1(4):333–339. doi: 10.1128/aac.1.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign J. C. Formation, properties, and germination of actinomycete spores. Annu Rev Microbiol. 1978;32:185–219. doi: 10.1146/annurev.mi.32.100178.001153. [DOI] [PubMed] [Google Scholar]
- Graham M. Y., Weisblum B. 23S ribosomal ribonucleic acid of macrolide-producing streptomycetes contains methylated adenine. J Bacteriol. 1979 Mar;137(3):1464–1467. doi: 10.1128/jb.137.3.1464-1467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder S. L., Streitfeld M. M. Inducible and constitutive resistance to macrolide antibiotics and lincomycin in clinically isolated strains of Streptococcus pyogenes. Antimicrob Agents Chemother. 1973 Sep;4(3):327–331. doi: 10.1128/aac.4.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Dahlberg J. E., Weisblum B. Structure of an inducibly methylatable nucleotide sequence in 23S ribosomal ribonucleic acid from erythromycin-resistant Staphylococcus aureus. Biochemistry. 1973 Jan 30;12(3):457–460. doi: 10.1021/bi00727a015. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic F., Capmau M. L., Abbe J., Cerceau C., Dublanchet A., Duval J. Plasmid mediated pristinamycin resistance: PH 1A, a pristinamycin 1A hydrolase. Ann Microbiol (Paris) 1977 Nov-Dec;128B(4):471–474. [PubMed] [Google Scholar]
- Malke H., Jacob H. E., Störl K. Characterization of the antibiotic resistance plasmid ERL1 from Streptococcus pyogenes. Mol Gen Genet. 1976 Mar 30;144(3):333–338. doi: 10.1007/BF00341732. [DOI] [PubMed] [Google Scholar]
- Pestka S., Vince R., LeMahieu R., Weiss F., Fern L., Unowsky J. Induction of erythromycin resistance in Staphyloccus aureus by erythromycin derivatives. Antimicrob Agents Chemother. 1976 Jan;9(1):128–130. doi: 10.1128/aac.9.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar A. G., Hahn J., Grandi G., Kozlov Y., Dubnau D. Posttranscriptional regulation of an erythromycin resistance protein specified by plasmic pE194. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3903–3907. doi: 10.1073/pnas.77.7.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Mutant of Staphylococcus aureus with lincomycin- and carbomycin-inducible resistance to erythromycin. Antimicrob Agents Chemother. 1974 May;5(5):538–540. doi: 10.1128/aac.5.5.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]