Abstract
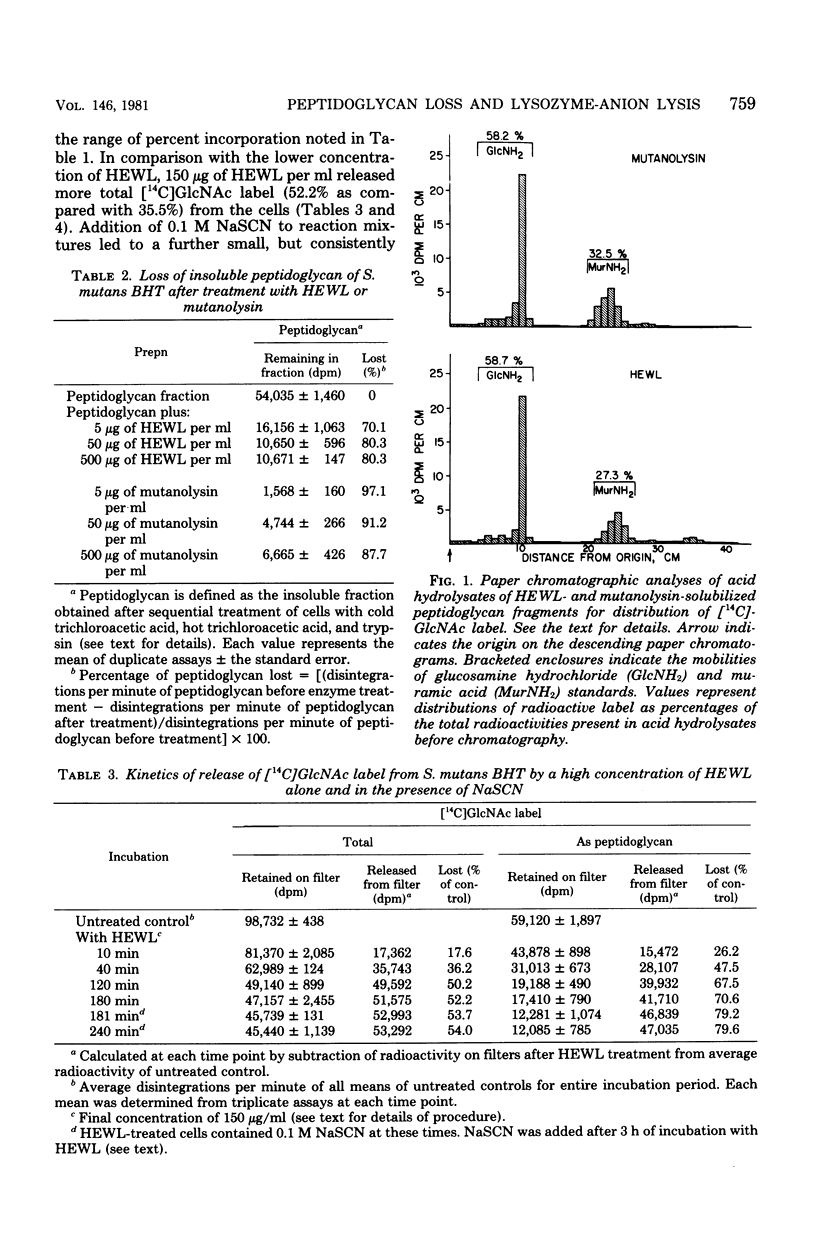
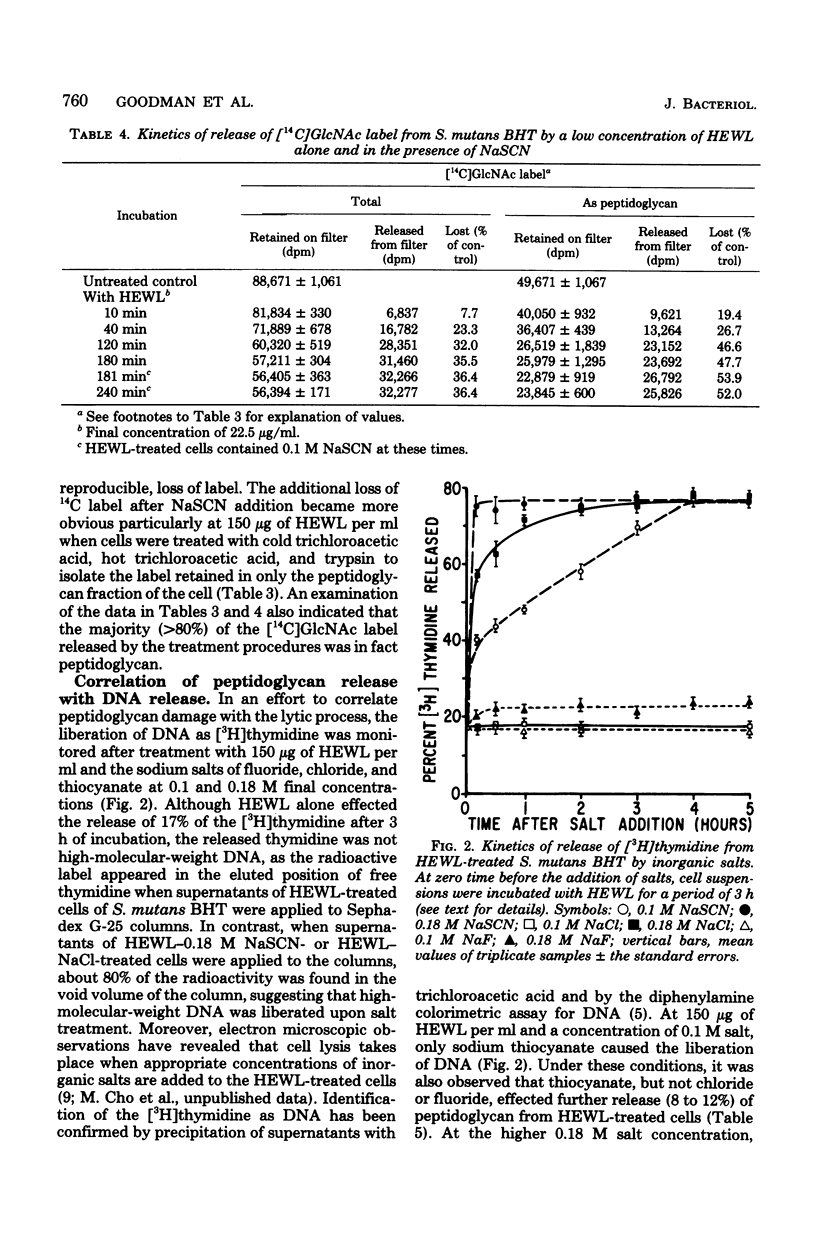
Streptococcus mutans BHT was grown in Todd-Hewitt dialysate medium containing N-acetyl[14C]glucosamine for 6 to 11 generations. After treatment with cold and hot trichloroacetic acid and trypsin, 52 to 65% of the radioactivity remained present in insoluble peptidoglycan-containing residues. Hen egg white lysozyme or mutanolysin treatment of the peptidoglycan residues resulted in the release of 80 and 97%, respectively, of the 14C label to the supernatant fraction. Hydrochloric acid hydrolysates of such supernatants showed that essentially all of the radioactivity present in insoluble peptidoglycan fractions was present in compounds that comigrated on paper chromatography with glucosamine (∼60%) or muramic acid (∼30%). Treatment of whole cells with low and high concentrations of lysozyme alone resulted in losses of 45 and 70% of the insoluble peptidoglycan, respectively, yet release of deoxyribonucleic acid from cells was not detected. Sequential addition of appropriate concentrations of selected inorganic salts after lysozyme treatment did result in the liberation of deoxyribonucleic acid. Deoxyribonucleic acid release was correlated with a further release of peptidoglycan from the insoluble fraction. However, the total amount of peptidoglycan lost effected by the low concentration of lysozyme and NaSCN (lysis) was significantly less than the amount of peptidoglycan hydrolyzed by high concentrations of lysozyme alone (no lysis), suggesting that the overall amount of peptidoglycan lost did not correlate well with cellular lysis. The total amount of insoluble peptidoglycan lost at the highest salt concentrations tested was found to be greater than could be accounted for by lysozyme-sensitive linkages of the peptidoglycan, possibly implicating autolysins. The results obtained suggested that hydrolysis of peptidoglycan bonds in topologically localized, but strategically important, sites was a more significant factor in the sequence that results in loss of cellular integrity (lysis).
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Fukuoka S., Oba S., Ito E. Enzymatic deacetylation of N-acetylglucosamine residues in peptidoglycan from Bacillus cereus cell walls. Biochem Biophys Res Commun. 1971 Nov 5;45(3):751–758. doi: 10.1016/0006-291x(71)90481-5. [DOI] [PubMed] [Google Scholar]
- BRUMFITT W. The mechanism of development of resistance to lysozyme by some gram-positive bacteria and its results. Br J Exp Pathol. 1959 Oct;40:441–451. [PMC free article] [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Lillmars K. A method for gentle lysis of Streptococcus sanguis and Streptococcus mutans. Biochem Biophys Res Commun. 1975 Jul 8;65(1):378–384. doi: 10.1016/s0006-291x(75)80104-5. [DOI] [PubMed] [Google Scholar]
- GRULA E. A., HARTSELL S. E. Lysozyme and morphological alterations induced in Micrococcus lysodeikticus. J Bacteriol. 1954 Aug;68(2):171–177. doi: 10.1128/jb.68.2.171-177.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis H. A., Miller S. E., Wheat R. W. Degradation of 14C-labeled streptococcal cell walls by egg white lysozyme and lysosomal enzymes. Infect Immun. 1976 May;13(5):1459–1466. doi: 10.1128/iai.13.5.1459-1466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick A. D., Ranhand J. M., Cole R. M. Degradation of group A streptococcal cell walls by egg-white lysozyme and human lysosomal enzymes. Infect Immun. 1972 Sep;6(3):403–413. doi: 10.1128/iai.6.3.403-413.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H., Pollock J. J., Katona L. I., Iacono V. J., Cho M. I., Thomas E. Lysis of Streptococcus mutans by hen egg white lysozyme and inorganic sodium salts. J Bacteriol. 1981 May;146(2):764–774. doi: 10.1128/jb.146.2.764-774.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Araki Y., Ito E. Occurrence of glucosamine residues with free amino groups in cell wall peptidoglycan from bacilli as a factor responsible for resistance to lysozyme. J Bacteriol. 1973 Feb;113(2):592–598. doi: 10.1128/jb.113.2.592-598.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden J. T., Grant W., Degroot J. Lysozyme sensitivity of pantothenate-deficient Lactobacillus plantarum grown with exogenous fatty acids. Biochim Biophys Acta. 1979 Jul 27;574(1):173–176. doi: 10.1016/0005-2760(79)90096-1. [DOI] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Lethal effect of a heterologous murein hydrolase on penicillin-treated Streptococcus sanguis. Antimicrob Agents Chemother. 1980 Feb;17(2):235–246. doi: 10.1128/aac.17.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono V. J., MacKay B. J., DiRienzo S., Pollock J. J. Selective antibacterial properties of lysozyme for oral microorganisms. Infect Immun. 1980 Aug;29(2):623–632. doi: 10.1128/iai.29.2.623-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., Campbell J. N. Effect of growth conditions on peptidoglycan structure and susceptibility to lytic enzymes in cell walls of Micrococcus sodonensis. Biochemistry. 1972 Jan 18;11(2):277–286. doi: 10.1021/bi00752a020. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., McDonald I. J. Peptidoglycan structure in cell walls of parental and filamentous Streptococcus cremoris HP. Can J Microbiol. 1974 Jul;20(7):905–913. doi: 10.1139/m74-140. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Holmwood K. J. Structure of the cell wall of Lactobacilli. Role of muramic acid phosphate in Lactobacillus fermenti. Biochem J. 1968 Jul;108(3):363–368. doi: 10.1042/bj1080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer R., Gill K., Slade H. D. Chemical composition of Streptococcus mutans type c antigen: comparison to type a, b, and d antigens. J Dent Res. 1976 Jan;55:A109–A115. doi: 10.1177/002203457605500103011. [DOI] [PubMed] [Google Scholar]
- Logardt I. M., Neujahr H. Y. Lysis of modified walls from Lactobacillus fermentum. J Bacteriol. 1975 Oct;124(1):73–77. doi: 10.1128/jb.124.1.73-77.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychajlonka M., McDowell T. D., Shockman G. D. Conservation of cell wall peptidoglycan by strains of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1980 Apr;28(1):65–73. doi: 10.1128/iai.28.1.65-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Pollock J. J., Sharon N. Studies on the acceptor specificity of the lysozyme-catalyzed transglycosylation reaction. Biochemistry. 1970 Sep 29;9(20):3913–3925. doi: 10.1021/bi00822a009. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. J. Cell wall of Micrococcus lysodeikticus as the substrate of lysozyme. Nature. 1952 Nov 1;170(4331):746–747. doi: 10.1038/170746a0. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. V. The action of lysozyme on cell walls of some lysozyme-sensitive bacteria. Biochim Biophys Acta. 1956 Dec;22(3):495–506. doi: 10.1016/0006-3002(56)90060-9. [DOI] [PubMed] [Google Scholar]
- SHARON N., SEIFTER S. A TRANSGLYCOSYLATION REACTION CATALYZED BY LYSOZYME. J Biol Chem. 1964 Jul;239:PC2398–PC2399. [PubMed] [Google Scholar]
- Vaught R. M., Bleiweis A. S. Antigens of Streptococcus mutans. II. Characterization of an antigen resembling a glycerol teichoic acid in walls of strain BHT. Infect Immun. 1974 Jan;9(1):60–67. doi: 10.1128/iai.9.1.60-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmacott D., Perkins H. R. Effects of lysozyme on Bacillus cereus 569: rupture of chains of bacteria and enhancement of sensitivity to autolysins. J Gen Microbiol. 1979 Nov;115(1):1–11. doi: 10.1099/00221287-115-1-1. [DOI] [PubMed] [Google Scholar]
- Work E. Factors affecting the susceptibility of bacterial cell walls to the action of lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):446–447. doi: 10.1098/rspb.1967.0045. [DOI] [PubMed] [Google Scholar]


