Abstract
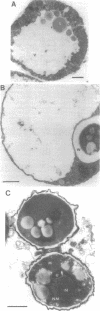
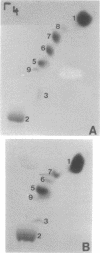
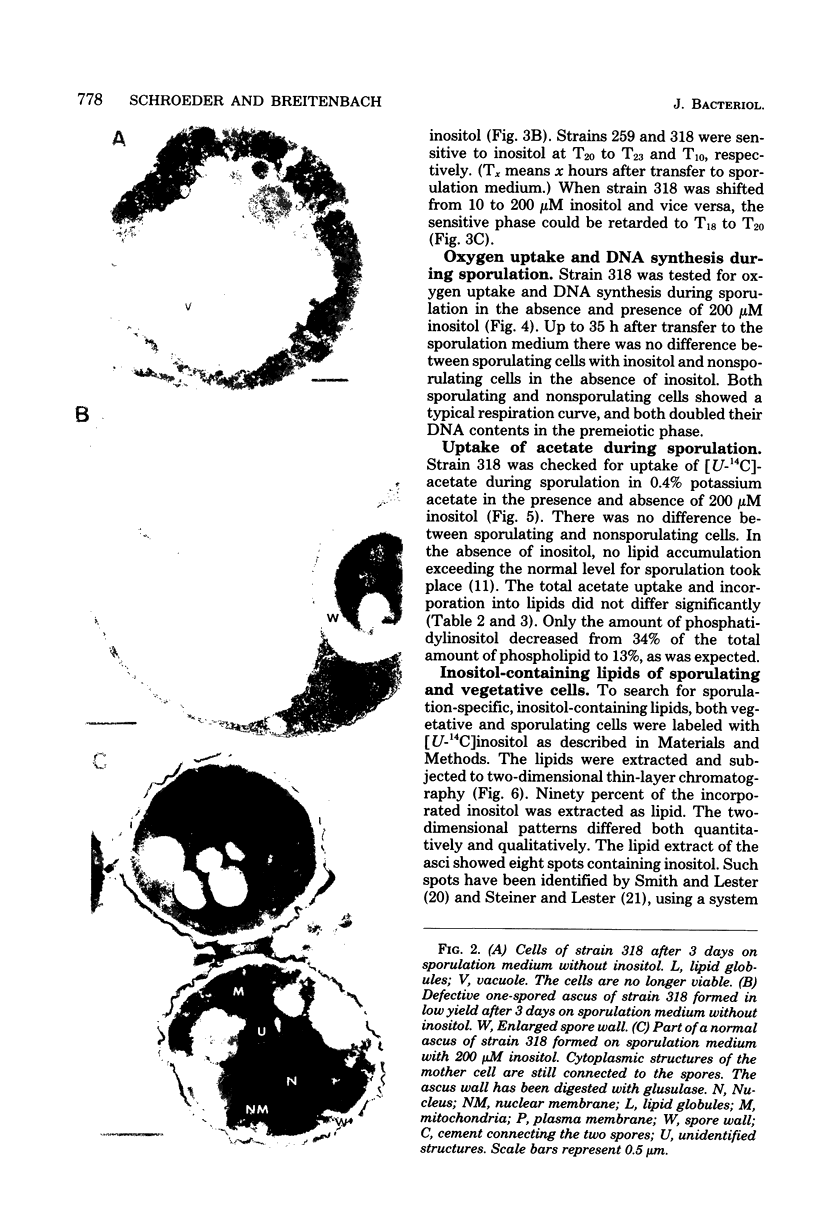
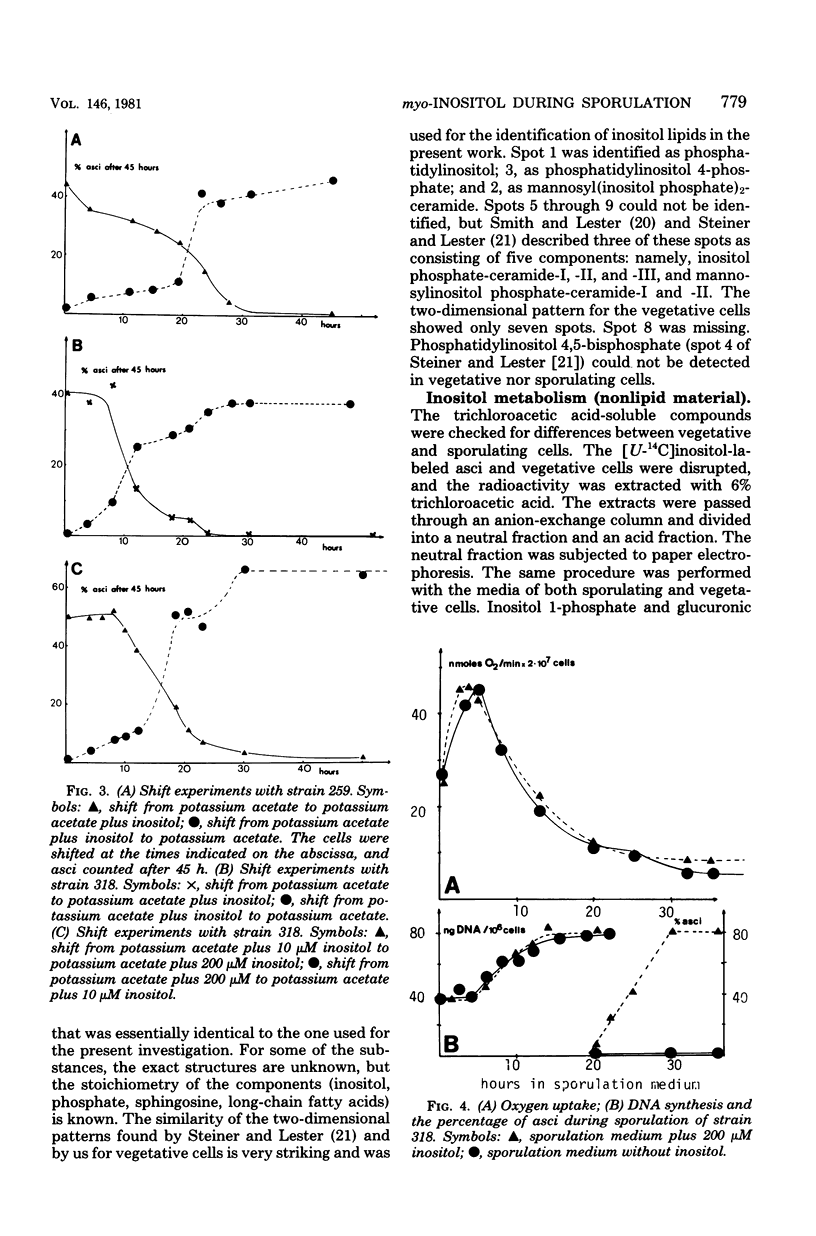
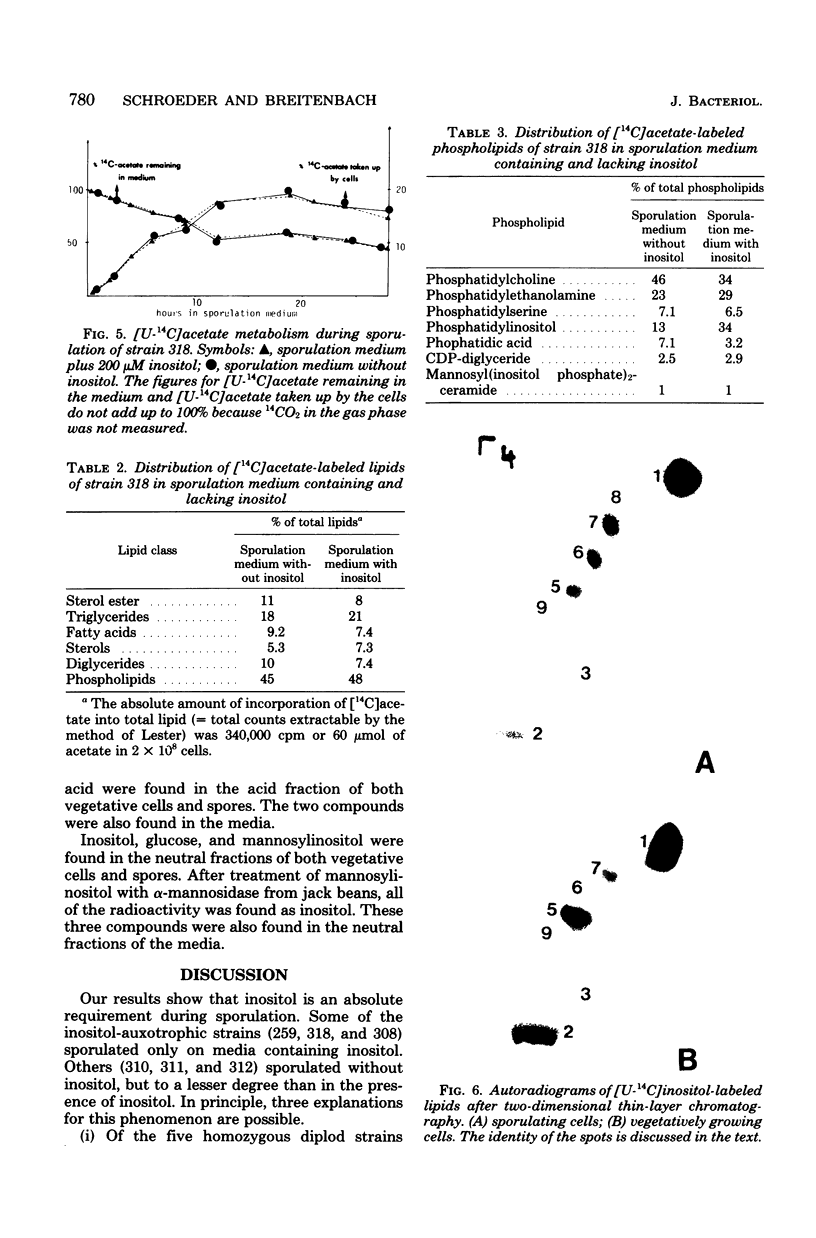
We investigated the sporulation properties of a series of diploid Saccharomyces cerevisiae strains homozygous for inositol auxotrophic markers. The strains required different amounts of inositol for the completion of sporulation. Shift experiments revealed two phases of inositol requirement during sporulation which coincided with the two phases of lipid synthesis found by earlier workers. Phase I was at the beginning and during premeiotic deoxyribonucleic acid synthesis; phase II immediately preceded the appearance of mature asci. Of the inositol taken up by sporulating cells, 90% was incorporated into inositol phospholipids. By two-dimensional thin-layer chromatography, eight compounds were resolved, one of which was sporulation specific. The majority of the inositol phospholipids were, however, identical to those found in vegetatively growing cells. In the absence of inositol, the cells did not sporulate but, after a certain time, were unable to return to vegetative growth. These nonsporulating cells did, however, incorporate acetate into lipids and double their deoxyribonucleic acid content in the premeiotic phase. We believe that it is this lack of coordination of biosynthetic events which causes inositol-less death on sporulation media without inositol.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus W. W., Lester R. L. Turnover of inositol and phosphorus containing lipids in Saccharomyces cerevisiae; extracellular accumulation of glycerophosphorylinositol derived from phosphatidylinositol. Arch Biochem Biophys. 1972 Aug;151(2):483–495. doi: 10.1016/0003-9861(72)90525-5. [DOI] [PubMed] [Google Scholar]
- Atkinson K. D., Kolat A. I., Henry S. A. Osmotic imbalance in inositol-starved spheroplasts of Saccharomyces cerevisiae. J Bacteriol. 1977 Dec;132(3):806–817. doi: 10.1128/jb.132.3.806-817.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G. W., Lester R. L. Changes in phospholipids of Saccharomyces cerevisiae associated with inositol-less death. J Biol Chem. 1977 Dec 10;252(23):8684–8691. [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae; inositol-phosphate synthetase mutants. J Bacteriol. 1976 Apr;126(1):243–250. doi: 10.1128/jb.126.1.243-250.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi W. A., Shanley M., Opheim D. J. Relationship of glycolytic intermediates, glycolytic enzymes, and ammonia to glycogen metabolism during sporulation in the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Jan;137(1):285–294. doi: 10.1128/jb.137.1.285-294.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek J., Wintersberger E. Glucose uptake in the cell cycle of Saccharomyces cerevisiae. Exp Cell Res. 1974 May;86(1):199–202. doi: 10.1016/0014-4827(74)90673-9. [DOI] [PubMed] [Google Scholar]
- Hayashi E., Hasegawa R., Tomita T. Accumulation of neutral lipids in Saccharomyces carlsbergensis by myo-inositol deficiency and its mechanism. Reciprocal regulation of yeast acetyl-CoA carboxylase by fructose bisphosphate and citrate. J Biol Chem. 1976 Sep 25;251(18):5759–5769. [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Halvorson H. O. Lipid synthesis during sporulation of Saccharomyces cerevisiae. J Bacteriol. 1973 Jun;114(3):1158–1163. doi: 10.1128/jb.114.3.1158-1163.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Keith A. D., Pollard E. C., Snipes W. Inositol-less death in yeast results in a simultaneous increase in intracellular viscosity. Biophys J. 1977 Mar;17(3):205–212. doi: 10.1016/S0006-3495(77)85650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P. Inositol deficiency resulting in death: an explanation of its occurrence in Neurospora crassa. Science. 1966 Jan 7;151(3706):86–88. doi: 10.1126/science.151.3706.86. [DOI] [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIVAK A., HOFFMANN-OSTENHOF O. [Enzymes of meso-ino-sitol decomposition in Schwanniomyces occidentalis]. Biochem Z. 1962;336:229–240. [PubMed] [Google Scholar]
- Smith S. W., Lester R. L. Inositol phosphorylceramide, a novel substance and the chief member of a major group of yeast sphingolipids containing a single inositol phosphate. J Biol Chem. 1974 Jun 10;249(11):3395–3405. [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J Bacteriol. 1972 Jan;109(1):81–88. doi: 10.1128/jb.109.1.81-88.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger M. W., Hartwell L. H. Control of cell division in Saccharomyces cerevisiae by methionyl-tRNA. Proc Natl Acad Sci U S A. 1976 May;73(5):1664–1668. doi: 10.1073/pnas.73.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]