Abstract
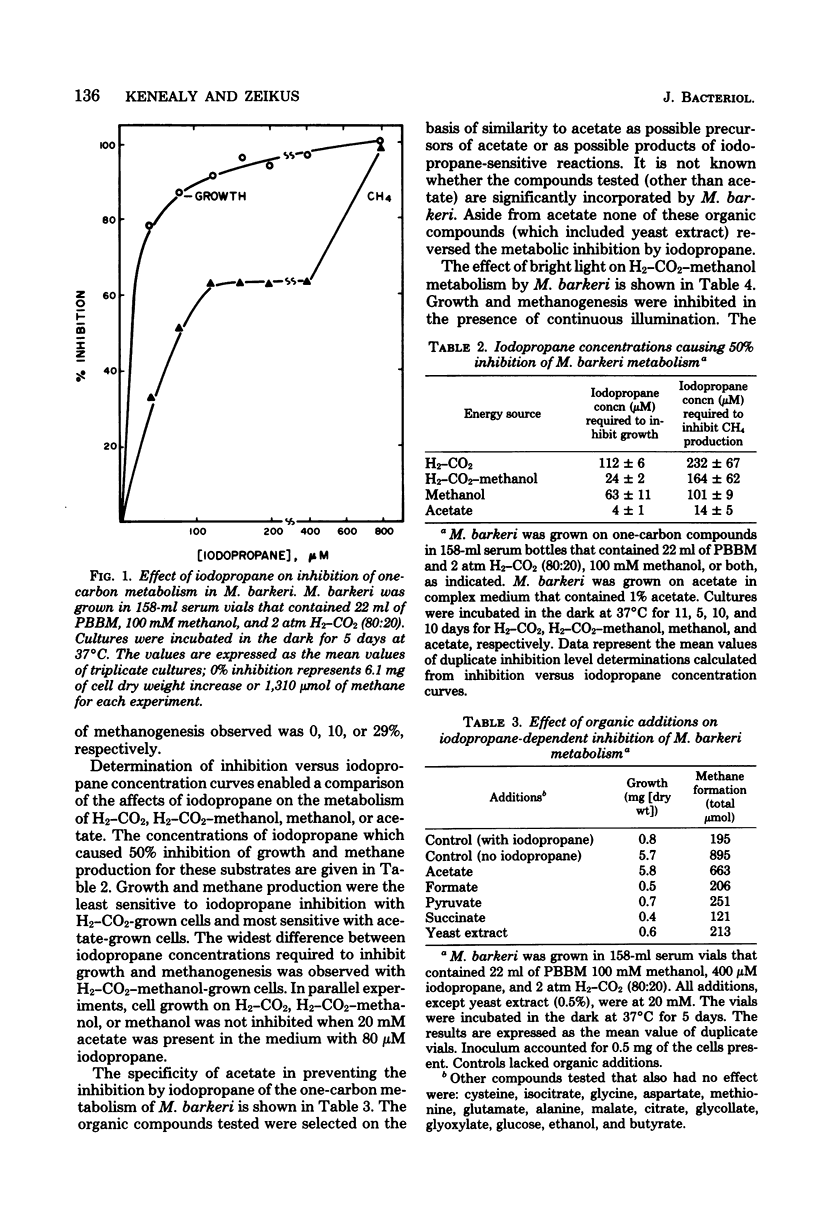
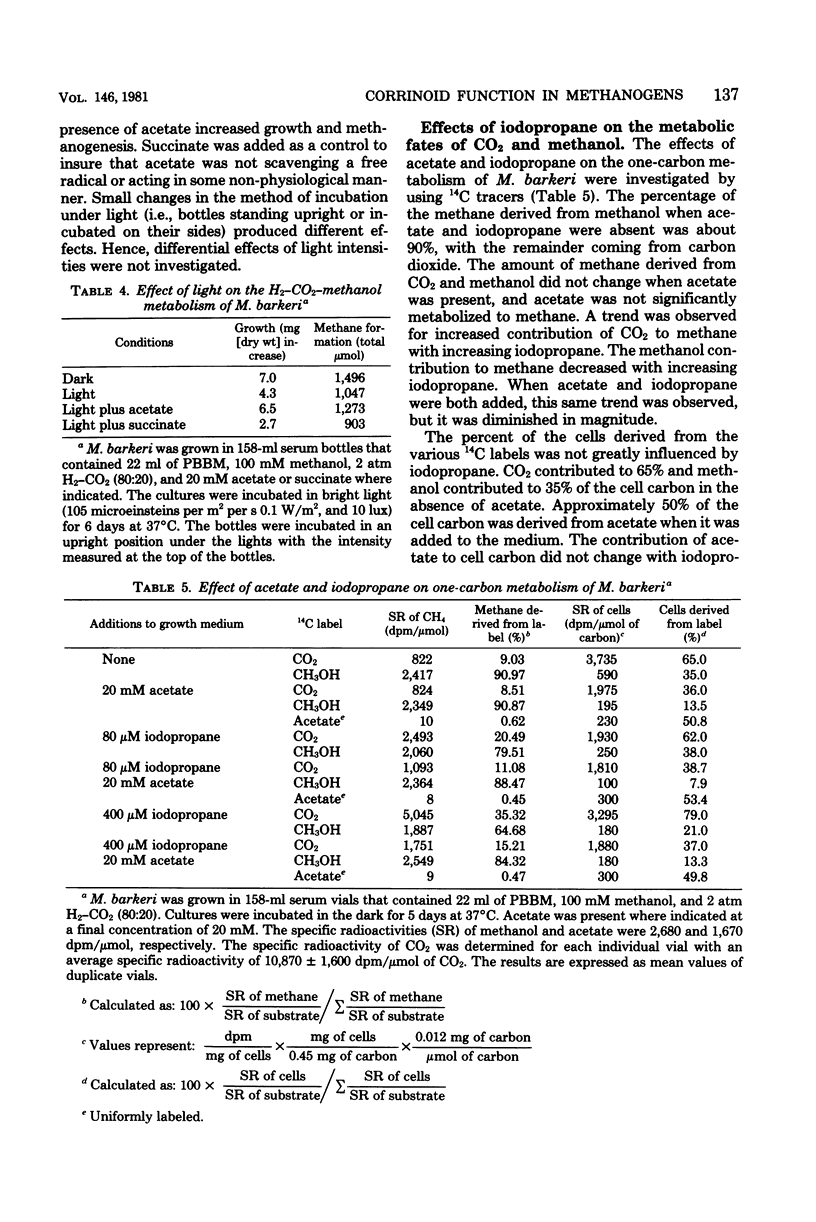
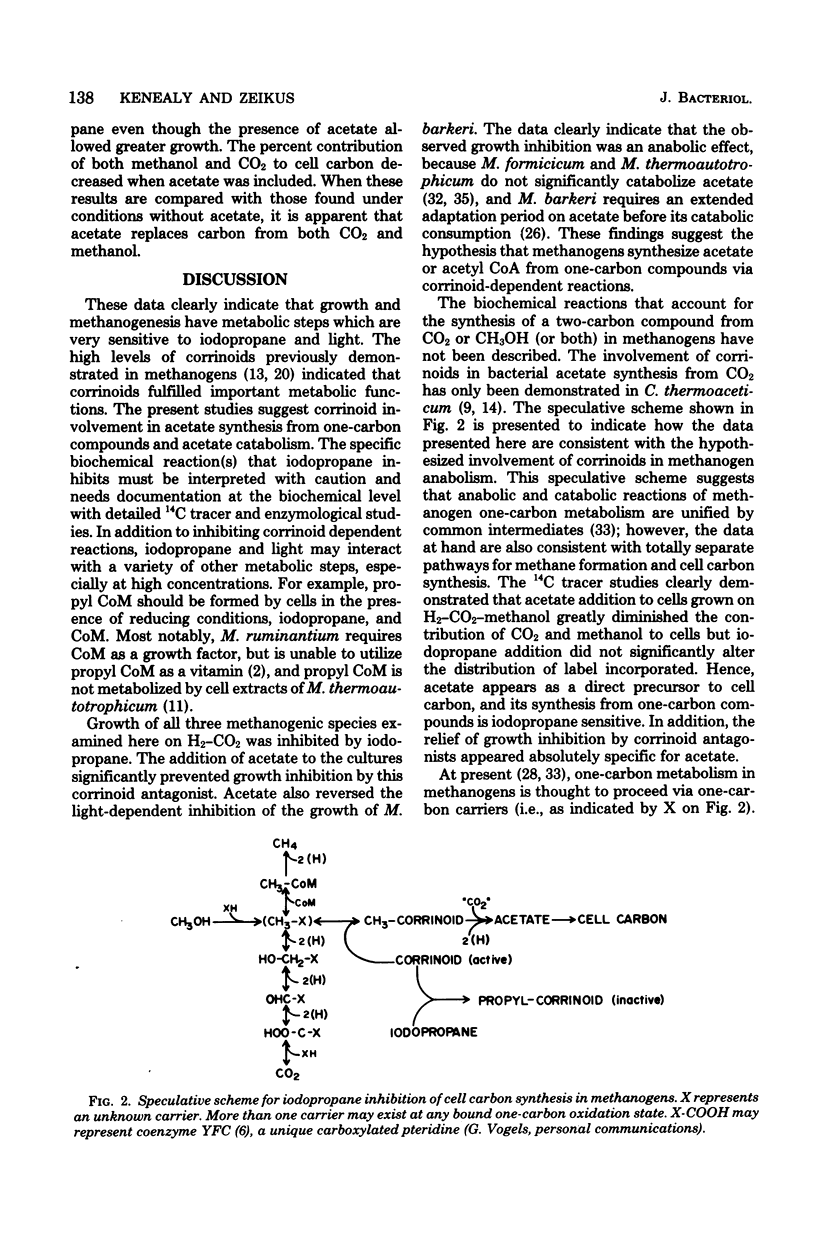
Iodopropane inhibited cell growth and methane production when Methanobacterium thermoautotrophicum, Methanobacterium formicicum, and Methanosarcina barkeri were cultured on H2-CO2. Iodopropane (40 microM) inhibited methanogenesis (30%) and growth (80%) when M. barkeri was cultured mixotrophically on H2-CO2-methanol. The addition of acetate to the medium prevented the observed iodopropane-dependent inhibition of growth. The concentrations of iodopropane that caused 50% inhibition of growth of M. barkeri on either H2-CO2, H2-CO2-methanol, methanol, and acetate were 112 +/- 6, 24 +/- 2, 63 +/- 11, and 4 +/- 1 microM, respectively. Acetate prevented the iodopropane-dependent inhibition of one-carbon metabolism. Cultivation of M. barkeri on H2-CO2-methanol in bright light also inhibited growth and methanogenesis to a greater extent in the absence than in the presence of acetate in the medium. Acetate was the only organic compound examined that prevented iodopropane-dependent inhibition of one-carbon metabolism in M. barkeri. The effect of iodopropane and acetate on the metabolic fates of methanol and carbon dioxide was determined with 14C tracers when M. barkeri was grown mixotrophically on H2-CO2-methanol. The addition of iodopropane decreased the contribution of methanol to methane and cell carbon while increasing the contribution of CO2 to cell carbon. Regardless of iodopropane, acetate addition decreased the contribution of methanol and CO2 to cell carbon without decreasing their contribution to methane. The corrinoid antagonists, light and iodopropane, appeared most specific for methanogen metabolic reactions involved in acetate synthesis from one-carbon compounds and acetate catabolism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Specificity and biological distribution of coenzyme M (2-mercaptoethanesulfonic acid). J Bacteriol. 1979 Jan;137(1):256–263. doi: 10.1128/jb.137.1.256-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A. Corrinoid-dependent enzymic reactions. Annu Rev Biochem. 1972;41:55–90. doi: 10.1146/annurev.bi.41.070172.000415. [DOI] [PubMed] [Google Scholar]
- Daniels L., Fuchs G., Thauer R. K., Zeikus J. G. Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol. 1977 Oct;132(1):118–126. doi: 10.1128/jb.132.1.118-126.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Zeikus J. G. One-carbon metabolism in methanogenic bacteria: analysis of short-term fixation products of 14CO2 and 14CH3OH incorporated into whole cells. J Bacteriol. 1978 Oct;136(1):75–84. doi: 10.1128/jb.136.1.75-84.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G., Stupperich E., Thauer R. K. Acetate assimilation and the synthesis of alanine, aspartate and glutamate in Methanobacterium thermoautotrophicum. Arch Microbiol. 1978 Apr 27;117(1):61–66. doi: 10.1007/BF00689352. [DOI] [PubMed] [Google Scholar]
- Ghambeer R. K., Wood H. G., Schulman M., Ljungdahl L. Total synthesis of acetate from CO2. 3. Inhibition by alkylhalides of the synthesis from CO2, methyltetrahydrofolate, and methyl-B12 by Clostridium thermoaceticum. Arch Biochem Biophys. 1971 Apr;143(2):471–484. doi: 10.1016/0003-9861(71)90232-3. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Hippe H., Caspari D., Fiebig K., Gottschalk G. Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by Methanosarcina barkeri. Proc Natl Acad Sci U S A. 1979 Jan;76(1):494–498. doi: 10.1073/pnas.76.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah R. A., Smith M. R., Baresi L. Studies on an acetate-fermenting strain of Methanosarcina. Appl Environ Microbiol. 1978 Jun;35(6):1174–1184. doi: 10.1128/aem.35.6.1174-1184.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. A new coenzyme of methyl transfer, coenzyme M. Biochemistry. 1971 Jun 8;10(12):2317–2324. doi: 10.1021/bi00788a022. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman M., Ghambeer R. K., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO2. VII. Evidence with Clostridium thermoaceticum that the carboxyl of acetate is derived from the carboxyl of pyruvate by transcarboxylation and not by fixation of CO2. J Biol Chem. 1973 Sep 25;248(18):6255–6261. [PubMed] [Google Scholar]
- Shapiro S., Wolfe R. S. Methyl-coenzyme M, an intermediate in methanogenic dissimilation of C1 compounds by Methanosarcina barkeri. J Bacteriol. 1980 Feb;141(2):728–734. doi: 10.1128/jb.141.2.728-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C., Blaylock B. A. Role of B12 compounds in methane formation. Fed Proc. 1966 Nov-Dec;25(6):1657–1661. [PubMed] [Google Scholar]
- Tanner R. S., Wolfe R. S., Ljungdahl L. G. Tetrahydrofolate enzyme levels in Acetobacterium woodii and their implication in the synthesis of acetate from CO2. J Bacteriol. 1978 May;134(2):668–670. doi: 10.1128/jb.134.2.668-670.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- Taylor R. T., Smucker L., Hanna M. L., Gill J. Aerobic photolysis of alkylcobalamins: quantum yields and light-action spectra. Arch Biochem Biophys. 1973 Jun;156(2):521–533. doi: 10.1016/0003-9861(73)90301-9. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate assimilation pathway of Methanosarcina barkeri. J Bacteriol. 1979 Jan;137(1):332–339. doi: 10.1128/jb.137.1.332-339.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate metabolism in Methanosarcina barkeri. Arch Microbiol. 1978 Nov 13;119(2):175–182. doi: 10.1007/BF00964270. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. One carbon metabolism in methanogenic bacteria. Cellular characterization and growth of Methanosarcina barkeri. Arch Microbiol. 1978 Oct 4;119(1):49–57. doi: 10.1007/BF00407927. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Wolfe R. S. Alkylation of an enzyme in the methane-forming system of Methanobacillus omelianskii. Biochem Biophys Res Commun. 1966 Jan 4;22(1):119–123. doi: 10.1016/0006-291x(66)90612-7. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Wolfe R. S. Propylation and purification of a B12 enzyme involved in methane formation. Biochemistry. 1966 Nov;5(11):3598–3603. doi: 10.1021/bi00875a031. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


