Abstract
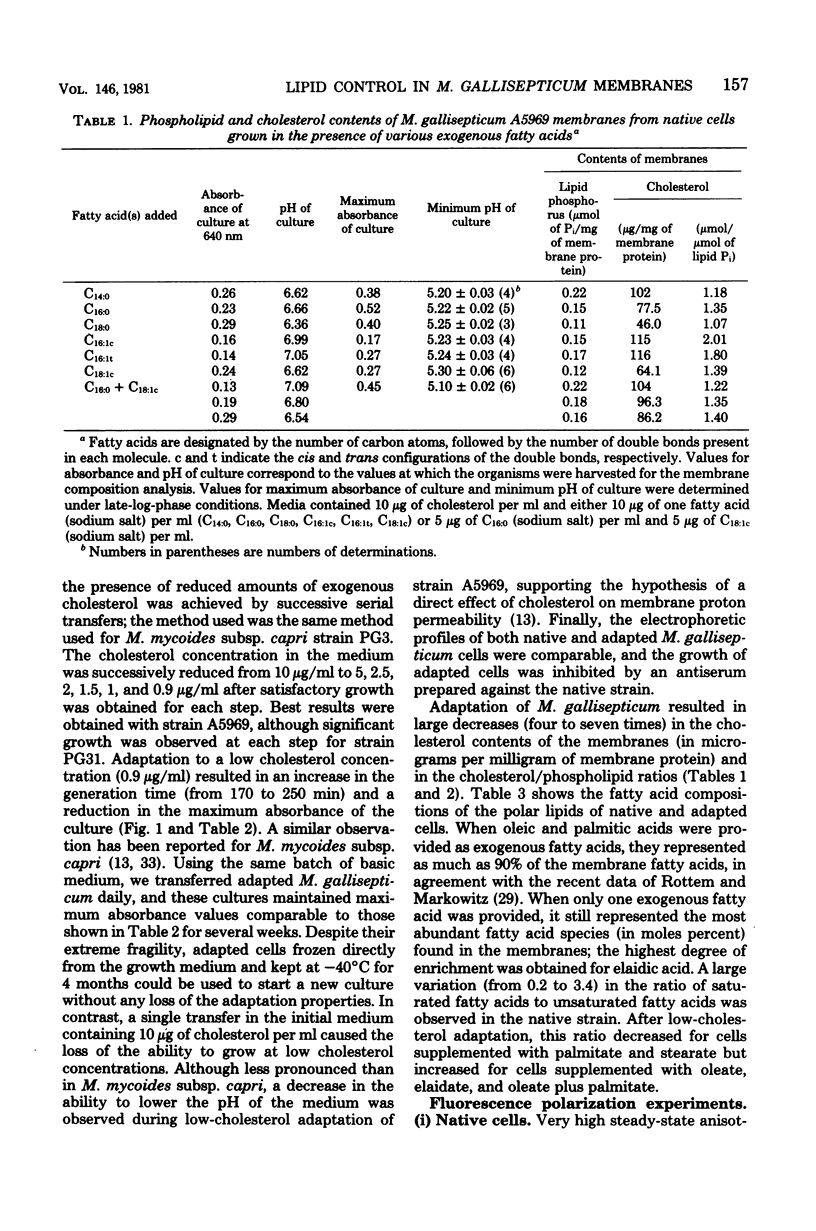
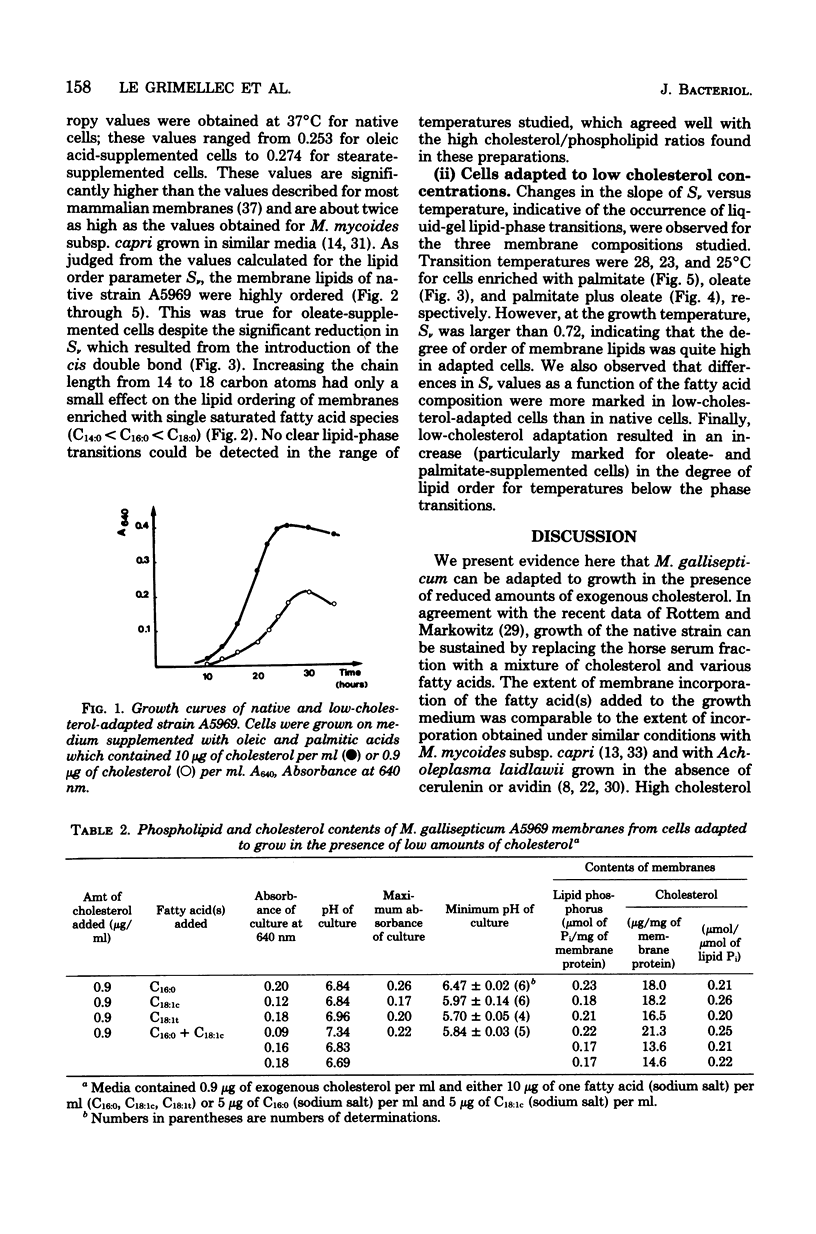
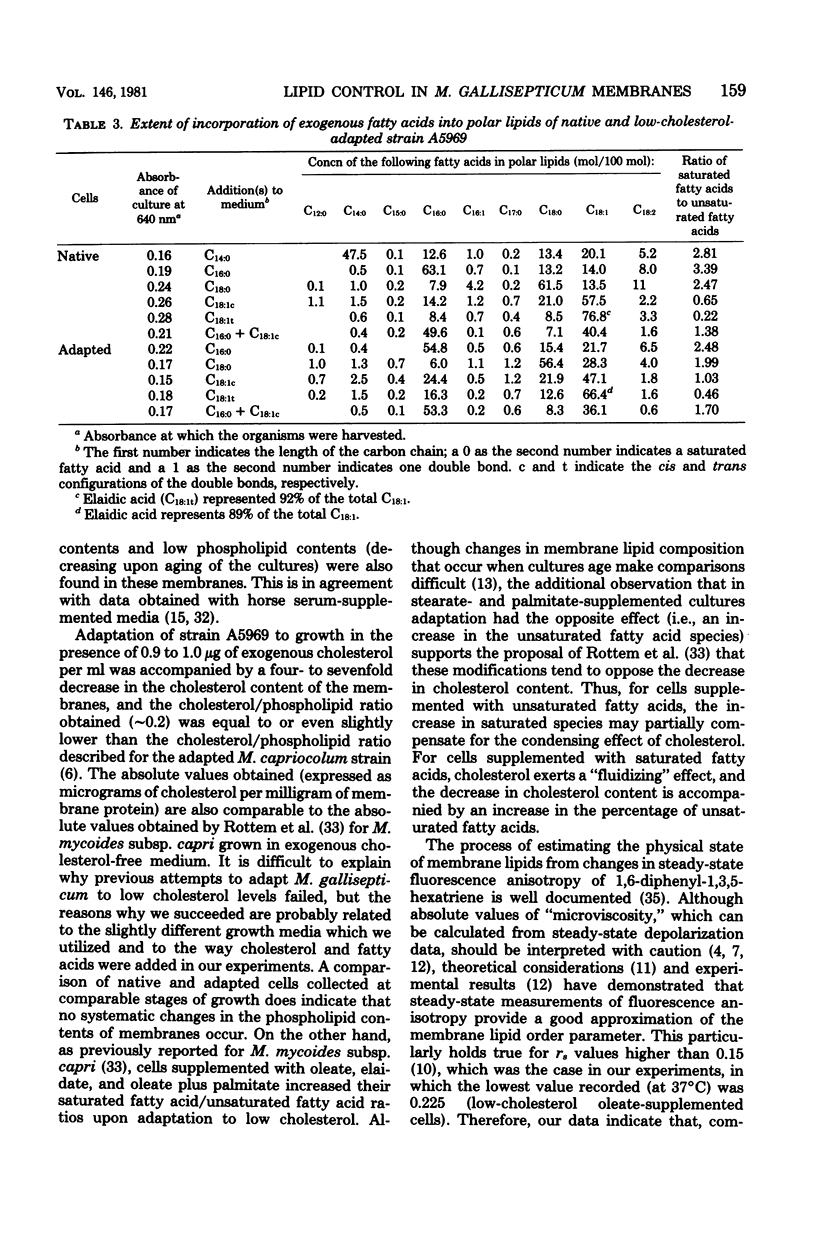
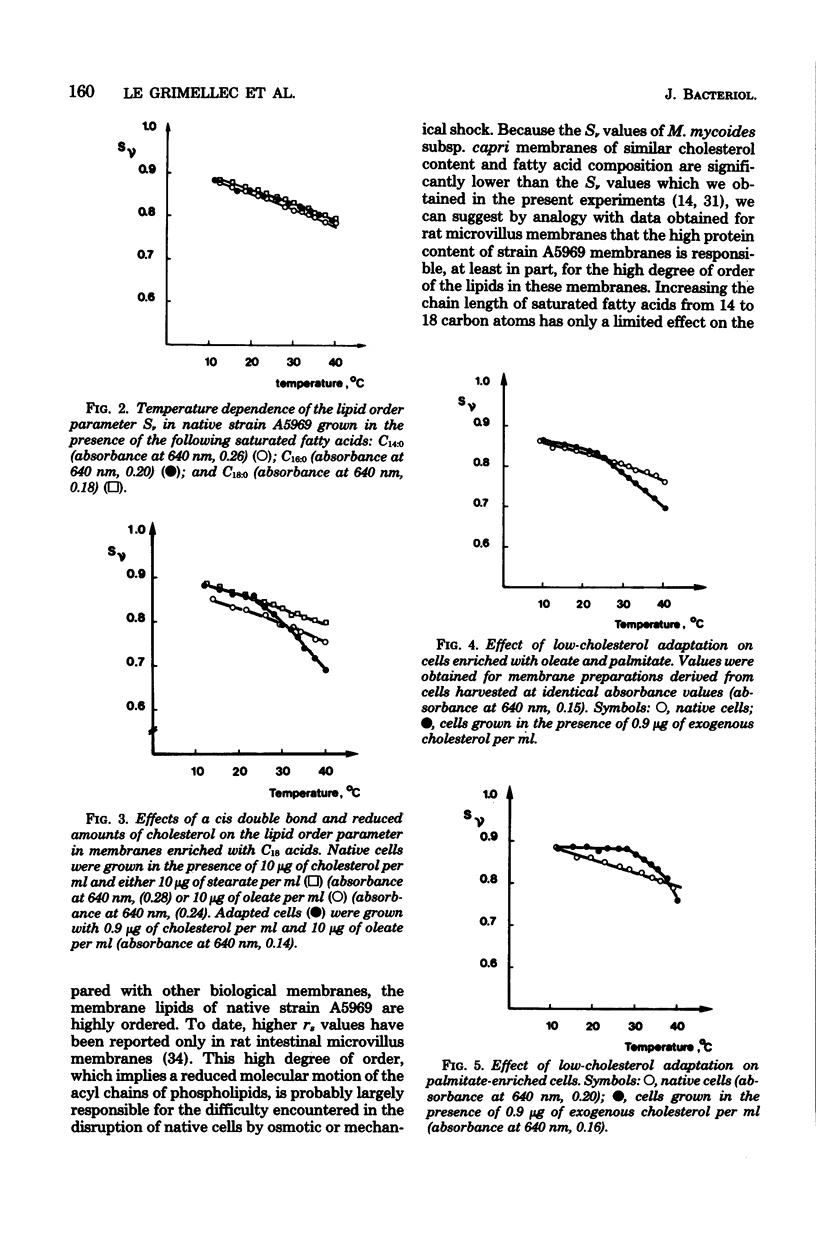
Adaptation of Mycoplasma gallisepticum, a sterol-requiring Mycoplasma sp., to growth in a serum-free medium supplemented with cholesterol in decreasing concentrations and with various saturated or unsaturated fatty acids enabled us to control both the cholesterol levels and the membrane fatty acid composition. An estimate of the membrane physical state from fluorescence polarization of 1,6-diphenyl-1,3,5-hexatriene indicated that the membrane lipids of native M. gallisepticum were highly ordered. Elongation of the saturated fatty acid chains from 14 to 18 carbon atoms caused only a small increase in the membrane lipid ordering, whereas the introduction of a cis double bond reduced it significantly. Lipid-phase transitions were observed in low-cholesterol-adapted organisms, whose membrane lipids were still highly ordered at the growth temperature.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARGAMAN M., RAZIN S. CHOLESTEROL AND CHOLESTEROL ESTERS IN MYCOPLASMA. J Gen Microbiol. 1965 Jan;38:153–160. doi: 10.1099/00221287-38-1-153. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- CHEN R. F., BOWMAN R. L. FLUORESCENCE POLARIZATION: MEASUREMENT WITH ULTRAVIOLET-POLARIZING FILTERS IN A SPECTROPHOTOFLUOROMETER. Science. 1965 Feb 12;147(3659):729–732. doi: 10.1126/science.147.3659.729. [DOI] [PubMed] [Google Scholar]
- Chen L. A., Dale R. E., Roth S., Brand L. Nanosecond time-dependent fluorescence depolarization of diphenylhexatriene in dimyristoyllecithin vesicles and the determination of "microviscosity". J Biol Chem. 1977 Apr 10;252(7):2163–2169. [PubMed] [Google Scholar]
- Clejan S., Bittman R., Rottem S. Uptake, transbilayer distribution, and movement of cholesterol in growing Mycoplasma capricolum cells. Biochemistry. 1978 Oct 31;17(22):4579–4583. doi: 10.1021/bi00615a001. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Chen L. A., Brand L. Rotational relaxation of the "microviscosity" probe diphenylhexatriene in paraffin oil and egg lecithin vesicles. J Biol Chem. 1977 Nov 10;252(21):7500–7510. [PubMed] [Google Scholar]
- Ghosh A., Das J., Maniloff J. Lack of repair of ultraviolet light damage in Mycoplasma gallisepticum. J Mol Biol. 1977 Oct 25;116(2):337–344. doi: 10.1016/0022-2836(77)90221-2. [DOI] [PubMed] [Google Scholar]
- Hildenbrand K., Nicolau C. Nanosecond fluorescence anisotropy decays of 1,6-diphenyl-1,3,5-hexatriene in membranes. Biochim Biophys Acta. 1979 Jun 2;553(3):365–377. doi: 10.1016/0005-2736(79)90292-x. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1977 May 31;16(11):2319–2324. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Grimellec C., Leblanc G. Effect of membrane cholesterol on potassium transport in Mycoplasma mycoides var. Capri (PG3). Biochim Biophys Acta. 1978 Dec 4;514(1):152–163. doi: 10.1016/0005-2736(78)90085-8. [DOI] [PubMed] [Google Scholar]
- Le Grimellec C., Leblanc G. Temperature-dependent relationship between K+ influx, Mg2+-ATPase activity, transmembrane potential and membrane lipid composition in mycoplasma. Biochim Biophys Acta. 1980 Jul;599(2):639–651. doi: 10.1016/0005-2736(80)90206-0. [DOI] [PubMed] [Google Scholar]
- Levisohn S., Razin S. Isolation, ultrastructure and antigenicity of Mycoplasma gallisepticum membranes. J Hyg (Lond) 1973 Dec;71(4):725–737. doi: 10.1017/s0022172400022981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANILOFF J., MOROWITZ H. J., BARRNETT R. J. STUDIES OF THE ULTRASTRUCTURE AND RIBOSOMAL ARRANGEMENTS OF THE PLEUROPNEUMONIA-LIKE ORGANISM A5969. J Cell Biol. 1965 Apr;25:139–150. doi: 10.1083/jcb.25.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOROWITZ H. J., TOURTELLOTTE M. E., GUILD W. R., CASTRO E., WOESE C. The chemical composition and submicroscopic morphology of Mycoplasma gallisepticum, avian PPLO 5969. J Mol Biol. 1962 Feb;4:93–103. doi: 10.1016/s0022-2836(62)80041-2. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J., Barrnett R. J. Ultrastructure and Ribosomes of Mycoplasma gallisepticum. J Bacteriol. 1965 Jul;90(1):193–204. doi: 10.1128/jb.90.1.193-204.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J. Cell biology of the mycoplasmas. Bacteriol Rev. 1972 Sep;36(3):263–290. doi: 10.1128/br.36.3.263-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney R. N., Tourtellotte M. E. Mycoplasma membrane lipids: variations in fatty acid composition. Science. 1969 Apr 25;164(3878):433–434. doi: 10.1126/science.164.3878.433. [DOI] [PubMed] [Google Scholar]
- Mcelhaney R. N., de Gier J., van der Neut-Kok E. C. The effect of alterations in fatty acid composition and cholesterol content on the nonelectrolyte permeability of Acholeplasma laidlawii B cells and derived liposomes. Biochim Biophys Acta. 1973 Mar 16;298(2):500–512. doi: 10.1016/0005-2736(73)90376-3. [DOI] [PubMed] [Google Scholar]
- PARSONS J., WYCOFF H. D. Chromatographic microassay for cholesterol and cholesterol esters. Science. 1957 Feb 22;125(3243):347–348. doi: 10.1126/science.125.3243.347. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Pollack M. E., Cleverdon R. C. Fractionation of mycoplasma cells for enzyme localization. Life Sci. 1965 May;4(9):973–977. doi: 10.1016/0024-3205(65)90200-6. [DOI] [PubMed] [Google Scholar]
- Quinlan D. C., Maniloff J. Membrane association of the deoxyribonucleic acid growing-point region in Mycoplasma gallisepticum. J Bacteriol. 1972 Dec;112(3):1375–1379. doi: 10.1128/jb.112.3.1375-1379.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S. FACTORS INFLUENCING OSMOTIC FRAGILITY OF MYCOPLASMA. J Gen Microbiol. 1964 Sep;36:451–459. doi: 10.1099/00221287-36-3-451. [DOI] [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Razin S., Cosenza B. J., Tourtellotte M. E. Variations in Mycoplasma morphology induced by long-chain fatty acids. J Gen Microbiol. 1966 Jan;42(1):139–145. doi: 10.1099/00221287-42-1-139. [DOI] [PubMed] [Google Scholar]
- Rottem S., Barile M. F. Effect of cerulenin on growth and lipid metabolism of mycoplasmas. Antimicrob Agents Chemother. 1976 Feb;9(2):301–307. doi: 10.1128/aac.9.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Cirillo V. P., de Kruyff B., Shinitzky M., Razin S. Cholesterol in mycoplasma membranes. Correlation of enzymic and transport activities with physical state of lipids in membranes of Mycoplasma mycoides var. capri adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):509–519. doi: 10.1016/0005-2736(73)90159-4. [DOI] [PubMed] [Google Scholar]
- Rottem S., Markowitz O. Membrane lipids of Mycoplasma gallisepticum: a disaturated phosphatidylcholine and a phosphatidylglycerol with an unusual positional distribution of fatty acids. Biochemistry. 1979 Jul 10;18(14):2930–2935. doi: 10.1021/bi00581a002. [DOI] [PubMed] [Google Scholar]
- Rottem S., Slutzky G. M., Bittman R. Cholesterol distribution and movement in the Mycoplasma gallisepticum cell membrane. Biochemistry. 1978 Jul 11;17(14):2723–2726. doi: 10.1021/bi00607a005. [DOI] [PubMed] [Google Scholar]
- Schachter D., Shinitzky M. Fluorescence polarization studies of rat intestinal microvillus membranes. J Clin Invest. 1977 Mar;59(3):536–548. doi: 10.1172/JCI108669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Dianoux A. C., Gitler C., Weber G. Microviscosity and order in the hydrocarbon region of micelles and membranes determined with fluorescent probes. I. Synthetic micelles. Biochemistry. 1971 May 25;10(11):2106–2113. doi: 10.1021/bi00787a023. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol. 1974 Jan 5;85(4):603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- TOURTELLOTTE M. E., JENSEN R. G., GANDER G. W., MOROWITZ H. J. LIPID COMPOSITION AND SYNTHESIS IN THE PLEUROPNEUMONIA-LIKE ORGANISM MYCOPLASMA GALLISEPTICUM. J Bacteriol. 1963 Sep;86:370–379. doi: 10.1128/jb.86.3.370-379.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- de Kruyff B., van Dijck P. W., Godlbach R. W., Demel R. A., van Deenen L. L. Influence of fatty acid and sterol composition on the lipid phase transition and activity of membrane-bound enzymes in Acholeplasma laidlawii. Biochim Biophys Acta. 1973 Dec 22;330(3):269–282. doi: 10.1016/0005-2736(73)90232-0. [DOI] [PubMed] [Google Scholar]


