Abstract
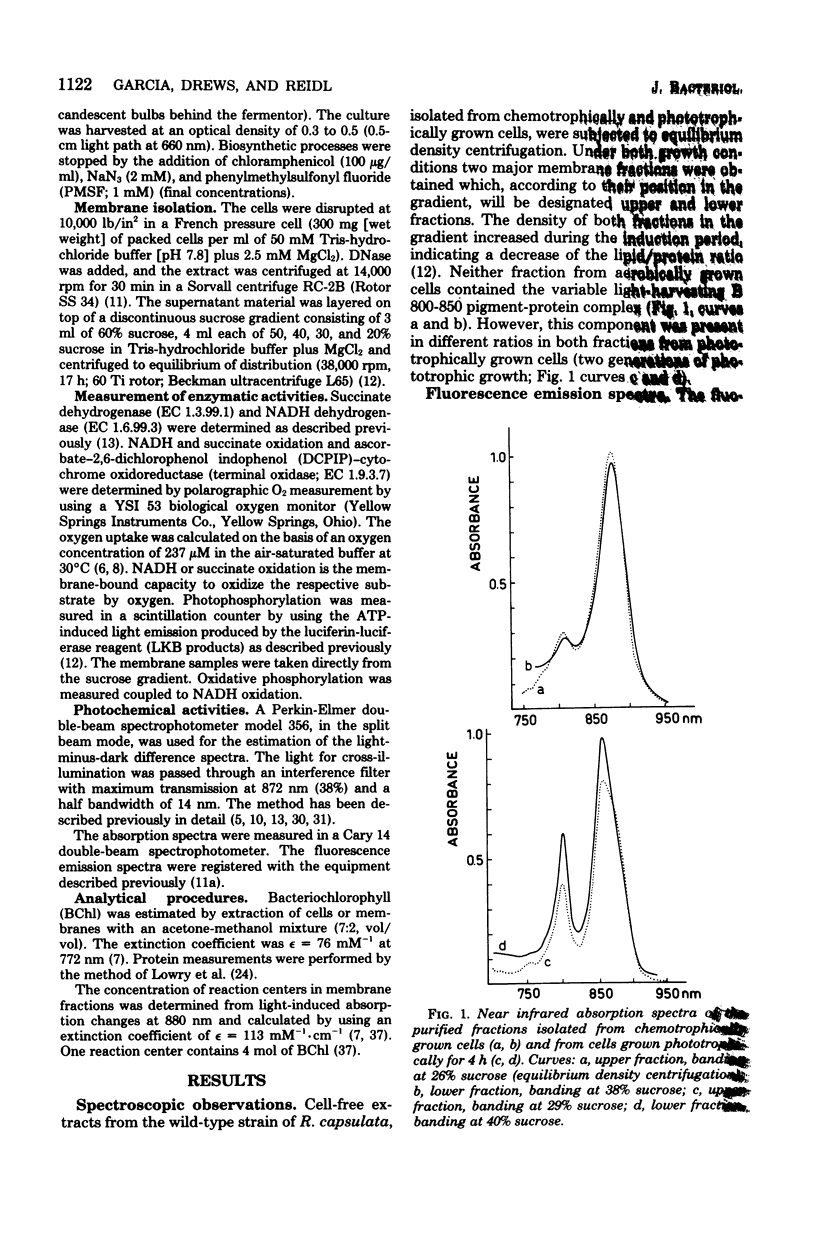
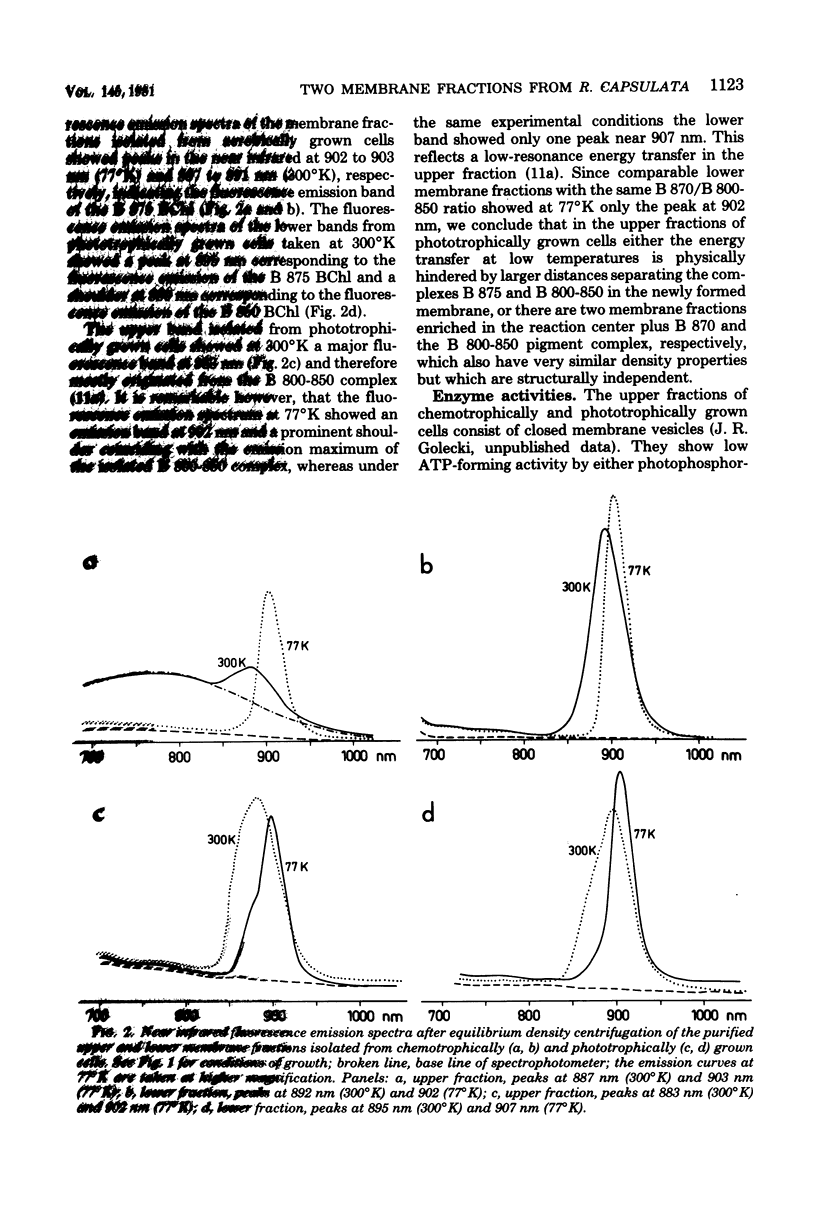
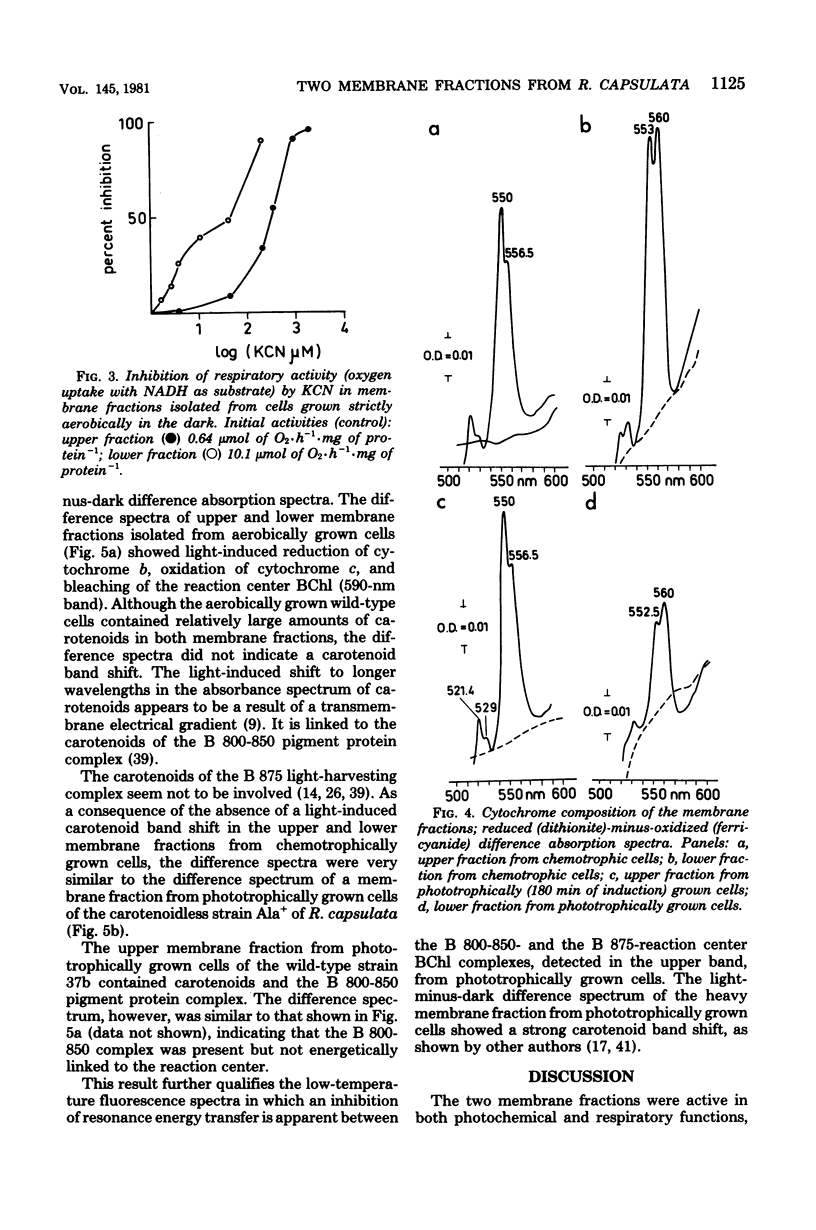
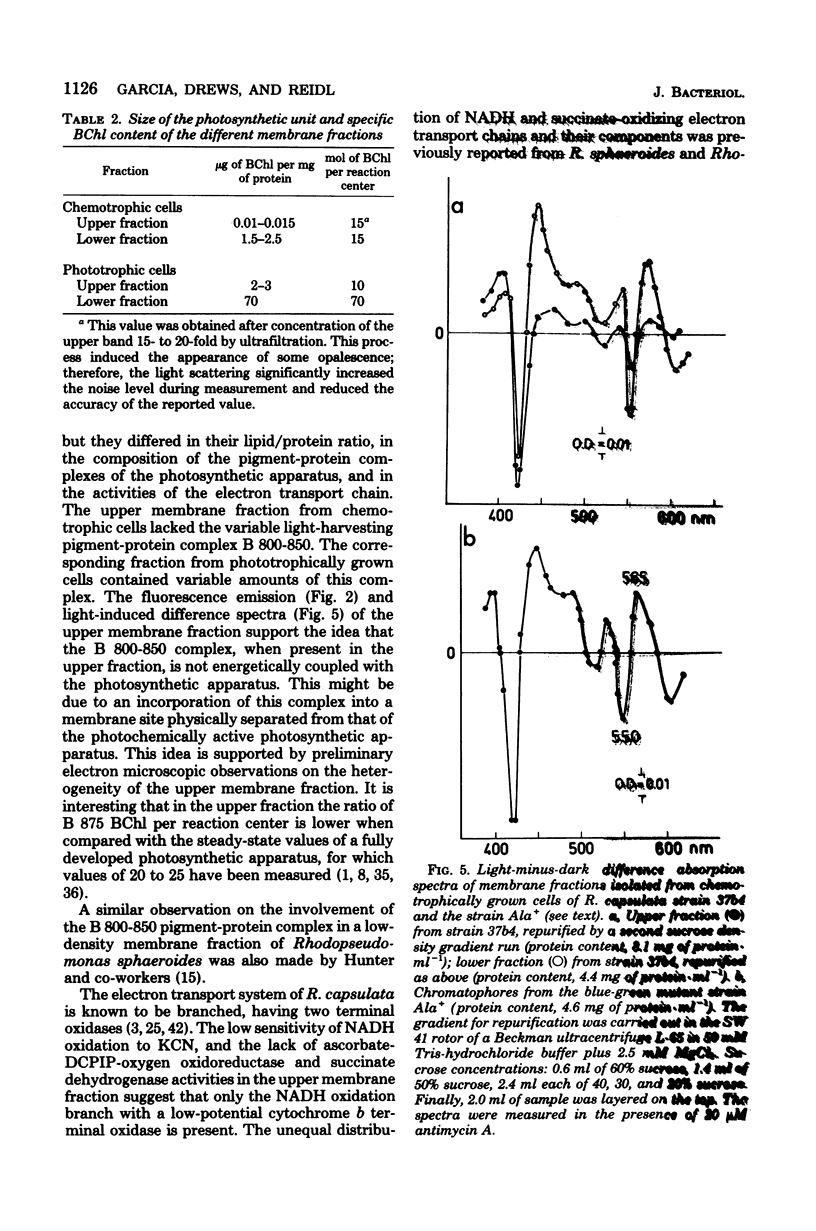
Light and heavy membrane fractions have been isolated by equilibrium sucrose density centrifugation from Rhodopseudomonas capsulata 938 GCM grown aerobically in the dark (chemotrophically) and anaerobically in the light (phototrophically). The densities of the light and heavy fractions from phototrophic cells were 1.1004 to 1.1006 and 1.1478, respectively, and the densities of the light and heavy fractions from chemotrophic cells were 1.0957 to 1.0958 and 1.1315, respectively. Both fractions were active in photochemical and respiratory functions and in electron transport-coupled phosphorylation. The light membrane fraction isolated from chemotrophic cells contained the reaction center and the light-harvesting pigment-protein complex B 870, but not the variable light-harvesting complex B 800-850. A small amount of the complex B 800-850 was present in the light fraction isolated from phototrophically grown cells, but it was not energetically coupled to the photosynthetic apparatus. From inhibitor studies, difference spectroscopy, and measurement of enzyme activities it was tentatively concluded that the light membrane fraction contains only the reduced nicotinamide adenine dinucleotide-oxidizing electron transport chain having a KCN-insensitive, low-potential cytochrome c oxidase, whereas the heavy fraction contains additionally the succinate dehydrogenase and a high-potential cytochrome b terminal oxidase sensitive to KCN. The light membrane fraction was more labile than the heavy fraction in terms of phosphorylating activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard J., Sistrom W. R. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972 Feb;15(2):209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- Baccarini Melandri A., Zannoni D., Melandri B. A. Energy transduction in photosynthetic bacteria. VI. Respiratory sites of energy conservation in membranes from dark-grown cells of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1973 Sep 26;314(3):298–311. doi: 10.1016/0005-2728(73)90114-x. [DOI] [PubMed] [Google Scholar]
- Barrett J., Hunter C. N., Jones O. T. Properties of a cytochrome c-enriched light particulate fraction isolated from the photosynthetic bacterium Rhodopseudomonas spheroides. Biochem J. 1978 Jul 15;174(1):267–275. doi: 10.1042/bj1740267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case G. D., Parson W. W., Thornber J. P. Photooxidation of cytochromes in reaction center preparations from Chromatium and Rhodopseudomonas viridis. Biochim Biophys Acta. 1970 Nov 3;223(1):122–128. doi: 10.1016/0005-2728(70)90137-4. [DOI] [PubMed] [Google Scholar]
- Cavari B. Z., Kalra V. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems: effect of chloramphenicol. J Bacteriol. 1971 Dec;108(3):1017–1025. doi: 10.1128/jb.108.3.1017-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierstein R., Drews G. Control of composition and activity of the photosynthetic apparatus of Rhodopseudomonas capsulata grown in ammonium-limited continuous culture. Arch Microbiol. 1975 Dec 31;106(3):227–235. doi: 10.1007/BF00446528. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Evans E. H., Crofts A. R. In situ characterisation of photosynthetic electron transport in Rhodopseudomonas capsulata. Biochim Biophys Acta. 1974 Jul 25;357(1):89–102. doi: 10.1016/0005-2728(74)90115-7. [DOI] [PubMed] [Google Scholar]
- Feick R., Drews G. Isolation and characterization of light harvesting bacteriochlorophyll.protein complexes from Rhodopseudomonas capsulata. Biochim Biophys Acta. 1978 Mar 13;501(3):499–513. doi: 10.1016/0005-2728(78)90117-2. [DOI] [PubMed] [Google Scholar]
- Feick R., van Grondelle R., Rijgersberg C. P., Drews G. Fluorescence emission by wild-type- and mutant-strains of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980 Dec 3;593(2):241–253. doi: 10.1016/0005-2728(80)90062-6. [DOI] [PubMed] [Google Scholar]
- Hunter C. N., van Grondelle R., Holmes N. G., Jones O. T., Niederman R. A. Fluorescence yield properties of a fraction enriched in newly synthesized bacteriochlorophyll a-protein complexes from rhodopseudomonas sphaeroides. Photochem Photobiol. 1979 Aug;30(2):313–316. doi: 10.1111/j.1751-1097.1979.tb07154.x. [DOI] [PubMed] [Google Scholar]
- Irschik H., Oelze J. The effect of transfer from low to high light intensity on electron transport in Rhodospirillum rubrum membranes. Arch Microbiol. 1976 Sep 1;109(3):307–313. doi: 10.1007/BF00446643. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R. The high energy state in chromatophores from Rhodopseudomonas spheroides. FEBS Lett. 1969 Aug;4(3):185–189. doi: 10.1016/0014-5793(69)80230-9. [DOI] [PubMed] [Google Scholar]
- Kennel S. J., Kamen M. D. Iron-containing proteins in Chromatium. I. Solubilization of membrane-bound cytochrome. Biochim Biophys Acta. 1971 Jun 15;234(3):458–467. doi: 10.1016/0005-2728(71)90212-x. [DOI] [PubMed] [Google Scholar]
- Kennel S. J., Kamen M. D. Iron-containing proteins in Chromatium. II. Purification and properties of cholate-solubilized cytochrome complex. Biochim Biophys Acta. 1971 Nov 2;253(1):153–166. doi: 10.1016/0005-2728(71)90241-6. [DOI] [PubMed] [Google Scholar]
- Klemme J. H., Schlegel H. G. Untersuchungen zum Cytochrom-Oxydase-System aus anaerob im Licht und aerob im Dunkeln gewachsenen Zellen von Rhodopseudomonas capsulata. Arch Mikrobiol. 1969;68(4):326–354. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampe H. H., Drews G. Die Differenzierung des Membransystems von Rhodopseudomonas capsulata hinsichtlich seiner photosynthetischen und respiratorischen Funktionen. Arch Mikrobiol. 1972;84(1):1–19. [PubMed] [Google Scholar]
- Lampe H. H., Oelze J., Drews G. Die Fraktionierung des Membransystems von Rhodopseudomonas capsulata und seine morphogenese. Arch Mikrobiol. 1972;83(1):78–94. [PubMed] [Google Scholar]
- Lien S., Gest H., San Pietro A. Regulation of chlorophyll synthesis in photosynthetic bacteria. J Bioenerg. 1973;4(4):423–434. doi: 10.1007/BF01648969. [DOI] [PubMed] [Google Scholar]
- Marrs B., Gest H. Genetic mutations affecting the respiratory electron-transport system of the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol. 1973 Jun;114(3):1045–1051. doi: 10.1128/jb.114.3.1045-1051.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K., Ishikawa T., Nishimura M. Correlation of membrane-potential-sensing carotenoid to pigment-protein complex II in Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1980 May 9;590(3):339–344. doi: 10.1016/0005-2728(80)90204-2. [DOI] [PubMed] [Google Scholar]
- Melandri A. B., Zannoni D. Photosynthetic and respiratory electron flow in the dual functional membrane of facultative photosynthetic bacteria. J Bioenerg Biomembr. 1978 Aug;10(3-4):109–138. doi: 10.1007/BF00743056. [DOI] [PubMed] [Google Scholar]
- Niederman R. A., Mallon D. E., Parks L. C. Membranes of Rhodopseudomonas sphaeroides. VI. Isolation of a fraction enriched in newly synthesized bacteriochlorophyll alpha-protein complexes. Biochim Biophys Acta. 1979 Aug 7;555(2):210–220. doi: 10.1016/0005-2736(79)90161-5. [DOI] [PubMed] [Google Scholar]
- OLSON J. M., CHANCE B. Oxidation-reduction reactions in the photosynthetic bacterium Chromatium. I. Absorption spectrum changes in whole cells. Arch Biochem Biophys. 1960 May;88:26–39. doi: 10.1016/0003-9861(60)90193-4. [DOI] [PubMed] [Google Scholar]
- OLSON J. M., CHANCE B. Oxidation-reduction reactions in the photosynthetic bacterium Chromatium. II. Dependence of light reactions on intensity of irradiation and quantum efficiency of cytochrome oxidation. Arch Biochem Biophys. 1960;88:40–53. doi: 10.1016/0003-9861(60)90194-6. [DOI] [PubMed] [Google Scholar]
- Oelze J., Kamen M. D. Separation of respiratory reactions in Rhodospirillum rubrum: inhibition studies with 2-hydroxydiphenyl. Biochim Biophys Acta. 1975 Apr 14;387(1):1–11. doi: 10.1016/0005-2728(75)90047-x. [DOI] [PubMed] [Google Scholar]
- Olson J. M., Nadler K. D. Energy transfer and cytochrome function in a new type of photosynthetic bacterium. Photochem Photobiol. 1965 Sep;4(4):783–791. doi: 10.1111/j.1751-1097.1965.tb07920.x. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Case G. D. In Chromatium, a single photochemical reaction center oxidizes both cytochrome C552 and cytochrome C555. Biochim Biophys Acta. 1970;205(2):232–245. doi: 10.1016/0005-2728(70)90253-7. [DOI] [PubMed] [Google Scholar]
- Schumacher A., Drews G. Effects of light intensity on membrane differentiation in Rhodopseudomonas capsulata. Biochim Biophys Acta. 1979 Sep 11;547(3):417–428. doi: 10.1016/0005-2728(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Schumacher A., Drews G. The formation of bacteriochlorophyll.protein complexes of the photosynthetic apparatus of Rhodopseudomonas capsulata during early stages of development. Biochim Biophys Acta. 1978 Feb 9;501(2):183–194. doi: 10.1016/0005-2728(78)90025-7. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Parson W. W., Mauzerall D. C., Clayton R. K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jun 28;305(3):597–609. doi: 10.1016/0005-2728(73)90079-0. [DOI] [PubMed] [Google Scholar]
- Thore A., Keister D. L., San Pietro A. Studies on the respiratory system of aerobically (dark) and anaerobically (light) grown Rhodospirillum rubrum. Arch Mikrobiol. 1969;67(4):378–396. doi: 10.1007/BF00412584. [DOI] [PubMed] [Google Scholar]
- Webster G. D., Cogdell R. J., Lindsay J. G. Identification of the carotenoid present in the B-800-850 antenna complex from Rhodopseudomonas capsulata as that which responds electrochromically to transmembrane electric fields. Biochim Biophys Acta. 1980 Jul 8;591(2):321–330. doi: 10.1016/0005-2728(80)90163-2. [DOI] [PubMed] [Google Scholar]
- Whale F. R., Jones O. T. The cytochrome system of heterotrophically-grown Rhodopseudomonas spheroides. Biochim Biophys Acta. 1970 Nov 3;223(1):146–157. doi: 10.1016/0005-2728(70)90139-8. [DOI] [PubMed] [Google Scholar]
- Zannoni D., Baccarini-Melandri A., Malandri B. A. Energy transduction in photosynthetic bacteria. The nature of cytochrome C oxidase in the respiratory chain of Rhodopseudomonas capsulata. FEBS Lett. 1974 Nov 1;48(1):152–155. doi: 10.1016/0014-5793(74)81085-9. [DOI] [PubMed] [Google Scholar]


