Abstract
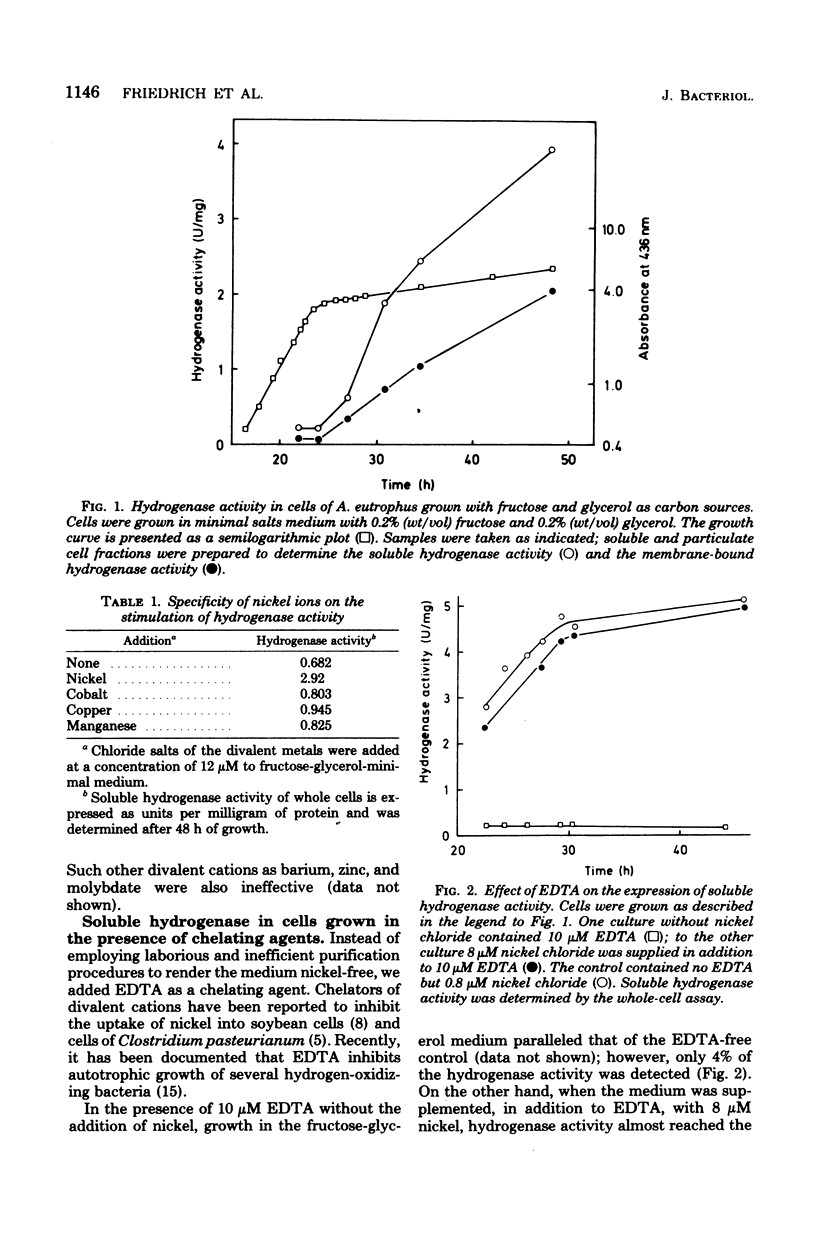
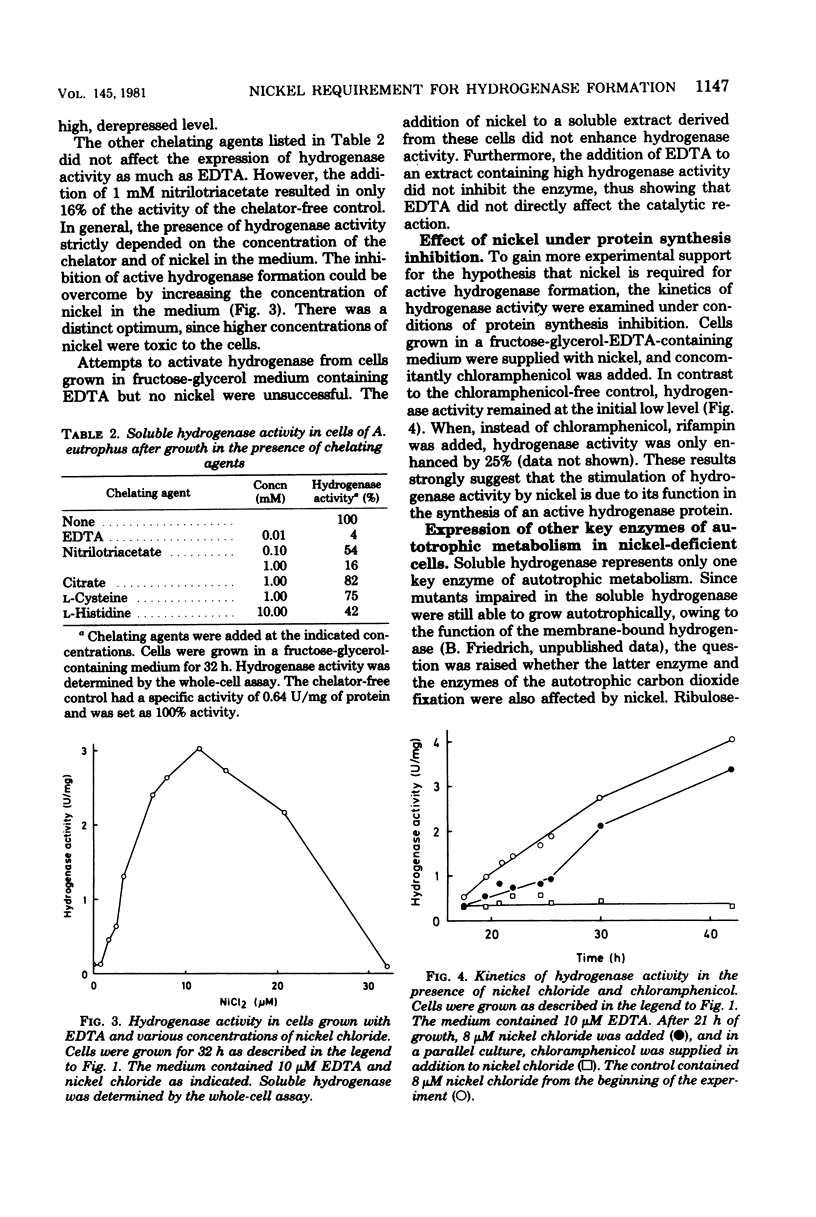
The nickel-dependent chemolithoautotrophic growth of Alcaligenes eutrophus is apparently due to a requirement of nickel for active hydrogenase formation. Cells grown heterotrophically with fructose and glycerol revealed a specific activity of soluble and membrane-bound hydrogenase which was severalfold higher than the normal autotrophic level. The omission of nickel from the medium did not affect heterotrophic growth, but the soluble hydrogenase activity was reduced significantly. In the presence of ethylenediaminetetraacetic acid (EDTA), almost no hydrogenase activity was detected. The addition of nickel allowed active hydrogenase formation even when EDTA was present. When chloramphenicol was added simultaneously with nickel to an EDTA-containing medium, almost no hydrogenase activity was found. This indicates that nickel ions are involved in a process which requires protein synthesis and not the direct reactivation of a preformed inactive protein. The formation of the membrane-bound hydrogenase also appeared to be nickel dependent. Autotrophic CO2 assimilation did not specifically require nickel ions, since formate was utilized in the presence of EDTA and the activity of ribulosebisphosphate carboxylase was not affected under these conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTHA R., ORDAL E. J. NICKEL-DEPENDENT CHEMOLITHOTROPHIC GROWTH OF TWO HYDROGENOMONAS STRAINS. J Bacteriol. 1965 Apr;89:1015–1019. doi: 10.1128/jb.89.4.1015-1019.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTHA R. [Physiological studies on the chemolithotropic metabolism of recently isolated Hydrogenomonas strains]. Arch Mikrobiol. 1962;41:313–350. [PubMed] [Google Scholar]
- Bowien B., Mayer F., Codd G. A., Schlegel H. G. Purification, some properties and quaternary structure of the D-ribulose 1,5-diphosphate carboxylase of Alcaligenes eutrophus. Arch Microbiol. 1976 Nov 2;110(23):157–166. doi: 10.1007/BF00690223. [DOI] [PubMed] [Google Scholar]
- Diekert G., Klee B., Thauer R. K. Nickel, a component of factor F430 from Methanobacterium thermoautotrophicum. Arch Microbiol. 1980 Jan;124(1):103–106. doi: 10.1007/BF00407036. [DOI] [PubMed] [Google Scholar]
- Polacco J. C. Nitrogen Metabolism in Soybean Tissue Culture: II. Urea Utilization and Urease Synthesis Require Ni. Plant Physiol. 1977 May;59(5):827–830. doi: 10.1104/pp.59.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus. I. Solubilization, purification, and biochemical properties. Biochim Biophys Acta. 1979 Apr 12;567(2):315–324. doi: 10.1016/0005-2744(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Schneider K., Cammack R., Schlegel H. G., Hall D. O. The iron-sulphur centres of soluble hydrogenase from Alcaligenes eutrophus. Biochim Biophys Acta. 1979 Jun 19;578(2):445–461. doi: 10.1016/0005-2795(79)90175-2. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Sperl G. T., DeMoss J. A. In vitro incorporation of molybdate into demolybdoproteins in Escherichia coli. J Bacteriol. 1979 Feb;137(2):719–726. doi: 10.1128/jb.137.2.719-726.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


