Abstract
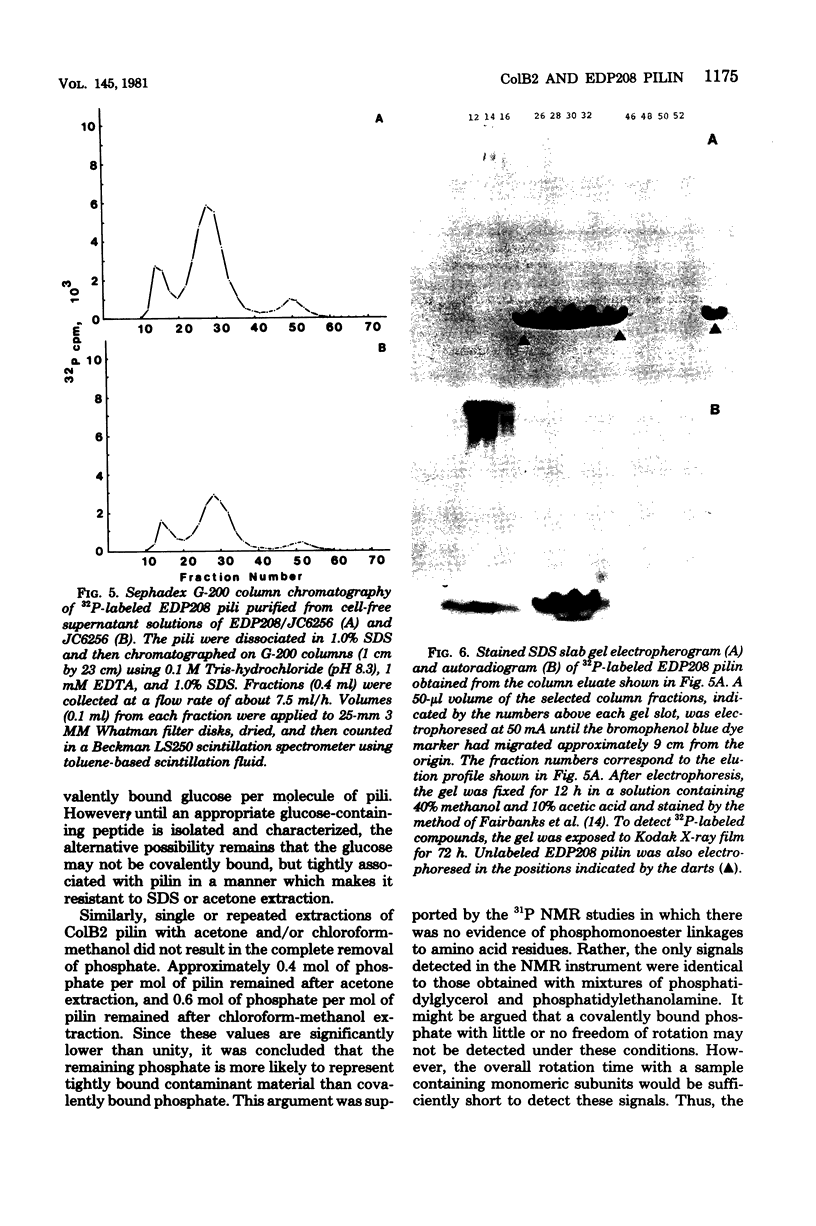
Studies were carried out to elucidate the nature of phosphate and sugar linkages to F-like conjugative pili encoded by the ColB2Fdr (compatibility group FII) and EDP208 (compatibility group FV) plasmids. Both types of pili, when in the intact undissociated state, were found to contain approximately 3 mol of phosphate and 3 mol of sugar per mol of pilin. However, further purification of the two types of pilin by gel filtration chromatography in the presence of sodium dodecyl sulfate (SDS) removed all of the carbohydrate from EDP208 pilin and approximately 65% of the carbohydrate from ColB2 pilin. Approximately 0.8 to 1.0 mol of glucose per mol of protein remained associated with ColB2 pilin after SDS gel filtration chromatography, but it was not possible to determine whether this was covalently linked to the pilin, or tightly associated in an SDS-resistant manner. SDS-gel chromatography did not remove phosphate from either ColB2 or EDP208 pilins. 31P nuclear magnetic resonance studies indicated that the pilin-associated phosphate is involved in a phosphodiester linkage. Acetone precipitation or chloroform-methanol extraction of the purified pilin material reduced the phosphate associated with EDP208 pilin to less than 0.04 molecule per pilin monomer. ColB2 pilin, under the same conditions, retained approximately 0.5 phosphate per pilin monomer. The extracted phosphate-containing moieties were identified as phosphatidylglycerol and phosphatidylethanolamine by thin-layer chromatography. Since the 31P nuclear magnetic resonance spectra for both ColB2 and EDP208 were identical and no signal other than that of a phosphodiester was detected in the ColB2 spectrum, the phosphate remaining with the ColB2 pilin after chloroform-methanol extraction is most likely due to a tightly bound noncovalent residue.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M. Mating aggregates in Escherichia coli conjugation. J Bacteriol. 1975 Aug;123(2):505–515. doi: 10.1128/jb.123.2.505-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. D., Frost L. S., Sastry P. A., Paranchych W. Comparative biochemical studies on F and EDP208 conjugative pili. J Bacteriol. 1980 Jan;141(1):333–341. doi: 10.1128/jb.141.1.333-341.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The properties of sex pili, the viral nature of "conjugal" genetic transfer systems, and some possible approaches to the control of bacterial drug resistance. CRC Crit Rev Microbiol. 1971 May;1(1):105–160. doi: 10.3109/10408417109104479. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Cohen S. M., Bárány M. Analysis with intact tissue with 31P NMR. Annu Rev Biophys Bioeng. 1979;8:1–25. doi: 10.1146/annurev.bb.08.060179.000245. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Dawson G., Hough L. The simultaneous estimation of 6-deoxy-L-galactose (L-fucose), D-mannose, D-galactose, 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine) and N-acetylneuraminic acid (sialic acid) in glycopeptides and glycoproteins. Biochim Biophys Acta. 1967 Nov 28;148(2):342–349. doi: 10.1016/0304-4165(67)90129-8. [DOI] [PubMed] [Google Scholar]
- Cozzone P. J., Jardetzky O. Phosphorus-31 Fourier transform nuclear magnetic resonance study of mononucleotides and dinucleotides. 1. Chemical shifts. Biochemistry. 1976 Nov 2;15(22):4853–4859. doi: 10.1021/bi00667a016. [DOI] [PubMed] [Google Scholar]
- Date T., Inuzuka M., Tomoeda M. Purification and characterization of F pili from Escherichia coli. Biochemistry. 1977 Dec 13;16(25):5579–5585. doi: 10.1021/bi00644a030. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., James T. L. Covalently bound non-coenzyme phosphorus residues in flavoproteins: 31P nuclear magnetic resonance studies of Azotobacter flavodoxin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3786–3789. doi: 10.1073/pnas.76.8.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Folkhard W., Leonard K. R., Malsey S., Marvin D. A., Dubochet J., Engel A., Achtman M., Helmuth R. X-ray diffraction and electron microscope studies on the structure of bacterial F pili. J Mol Biol. 1979 May 15;130(2):145–160. doi: 10.1016/0022-2836(79)90423-6. [DOI] [PubMed] [Google Scholar]
- Frydman A., Meynell E. Interactions between de-repressed F-like R factors and wild type colicin B factors: superinfection immunity and repressor susceptibility. Genet Res. 1969 Dec;14(3):315–322. doi: 10.1017/s0016672300002135. [DOI] [PubMed] [Google Scholar]
- Hausmann C., Clowes R. C. ColB2-K77, a fertility-repressed F-like factor. J Bacteriol. 1971 Sep;107(3):900–906. doi: 10.1128/jb.107.3.900-906.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. S., Bayley H., Khorana H. G. Delipidation of bacteriorhodopsin and reconstitution with exogenous phospholipid. Proc Natl Acad Sci U S A. 1980 Jan;77(1):323–327. doi: 10.1073/pnas.77.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Reading C. L., Penhoet E. E., Ballou C. E. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [PubMed] [Google Scholar]
- Willetts N., Achtman M. Genetic analysis of transfer by the Escherichia coli sex factor F, using P1 transductional complementation. J Bacteriol. 1972 Jun;110(3):843–851. doi: 10.1128/jb.110.3.843-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



