Abstract
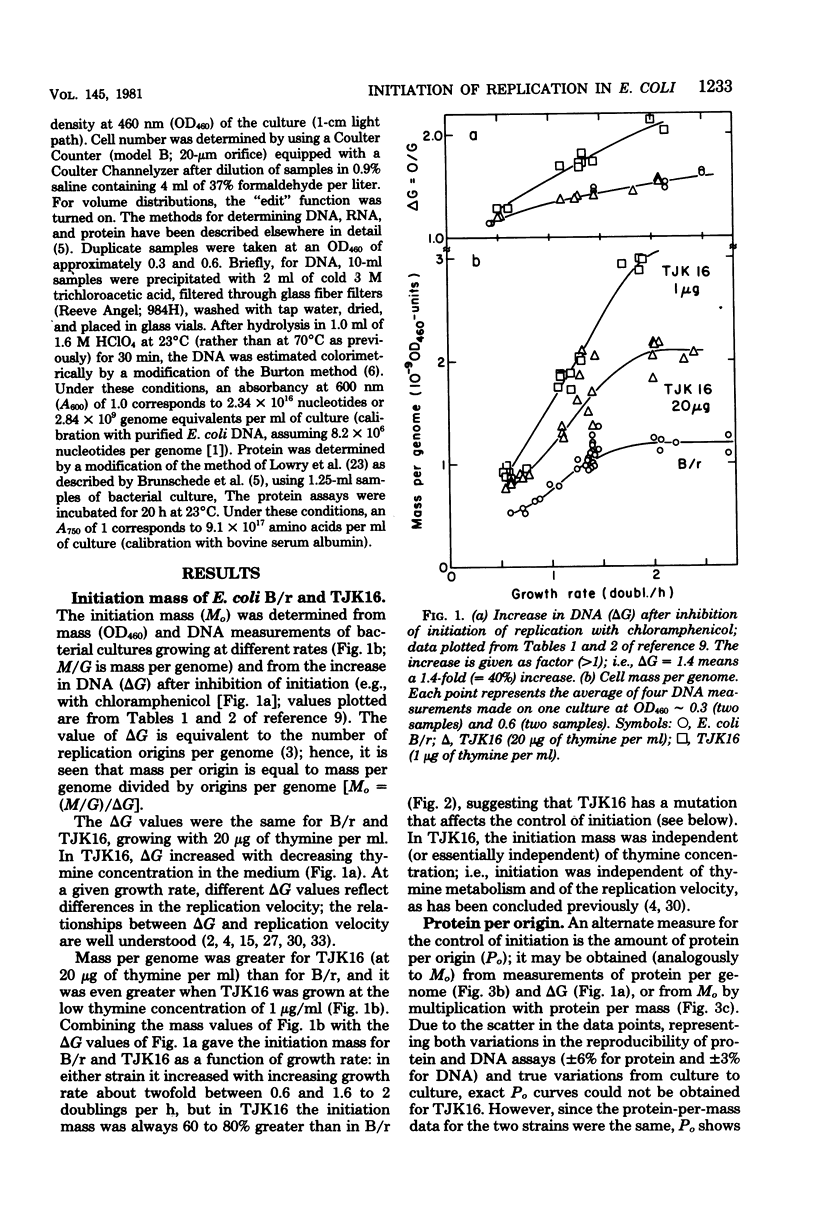
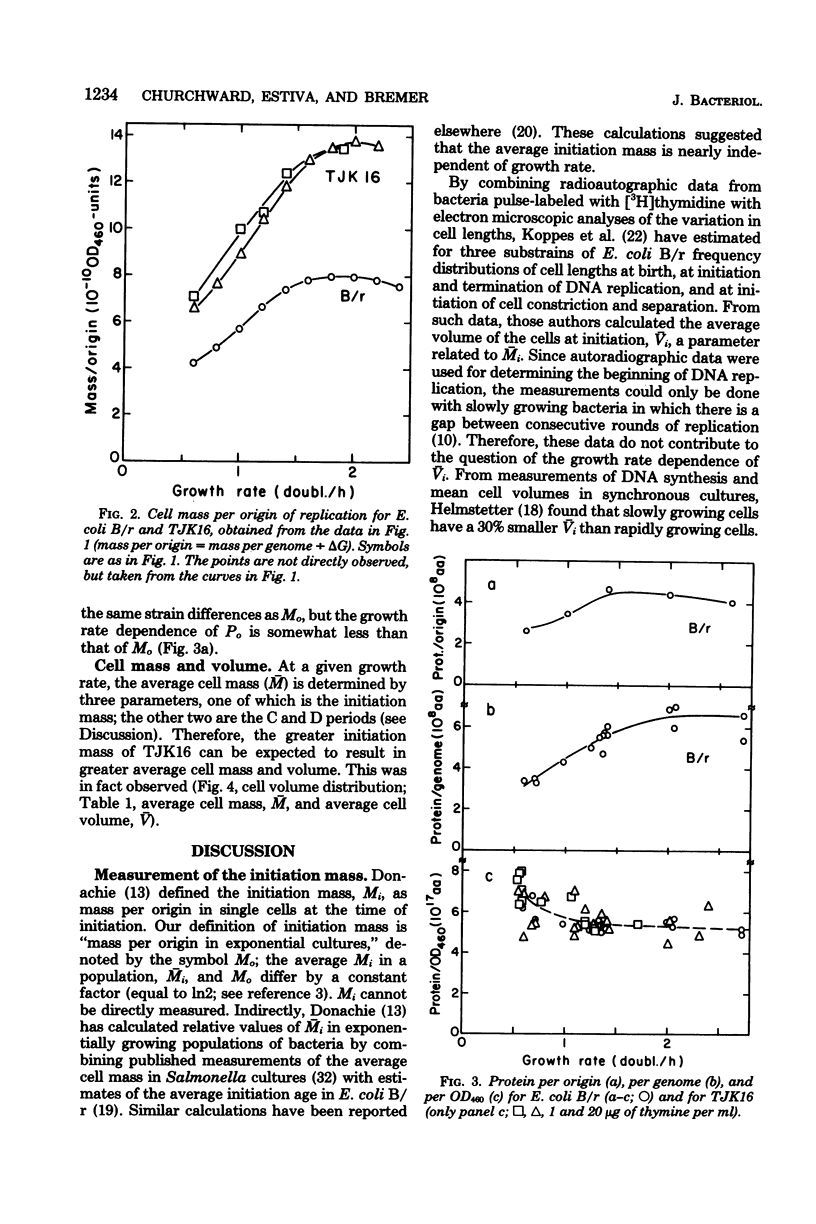
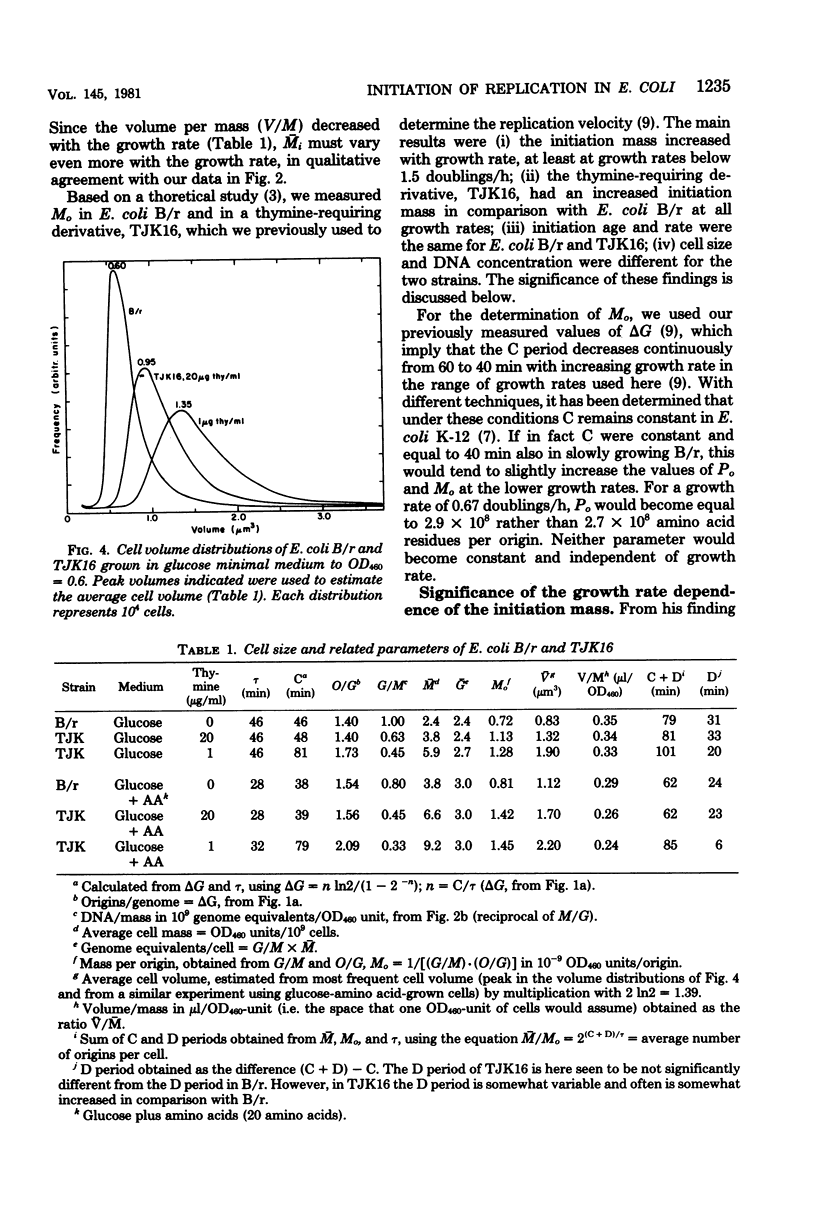
The initiation mass, defined as cell mass per origin of deoxyribonucleic acid replication (optical density units at 460 nm of culture/origins per milliliter of culture), reflects the intracellular concentration or activity of a hypothetical factor that controls initiation of chromosome replication in bacteria. In Escherichia coli B/r, the initiation mass was found to increase about twofold with increasing growth rate between 0.6 and 1.6 doublings per h; at higher growth rates it remained essentially constant (measured up to 2.4 doublings per h). A low-thymine-requiring (thyA deoB) derivative of E. coli B/r, strain TJK16, was found to have a 60 to 80% greater initiation mass than B/r which was independent of the replication velocity and not related to the thyA and deoB mutations. It is suggested that TJK16 had acquired, during its isolation, a mutation in a gene affecting the initiation of deoxyribonucleic acid replication. The initiation age was not altered by this mutation, but other parameters, including deoxyribonucleic acid concentration and cell size, were changed in comparison with the B/r parent, as expected from theoretical considerations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Churchward G. Deoxyribonucleic acid synthesis after inhibition of initiation of rounds of replication in Escherichia coli B/r. J Bacteriol. 1977 May;130(2):692–697. doi: 10.1128/jb.130.2.692-697.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Churchward G., Young R. Relation between growth and replication in bacteria. J Theor Biol. 1979 Dec 7;81(3):533–545. doi: 10.1016/0022-5193(79)90051-1. [DOI] [PubMed] [Google Scholar]
- Bremer H., Young R., Churchward G. Initiation and termination of deoxyribonucleic acid replication in bacteria after a stepwise increase in the velocity of replication. J Bacteriol. 1977 Apr;130(1):92–99. doi: 10.1128/jb.130.1.92-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunschede H., Dove T. L., Bremer H. Establishment of exponential growth after a nutritional shift-up in Escherichia coli B/r: accumulation of deoxyribonucleic acid, ribonucleic acid, and protein. J Bacteriol. 1977 Feb;129(2):1020–1033. doi: 10.1128/jb.129.2.1020-1033.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M., Bird R. E., Caro L. The replication time of the Escherichia coli K12 chromosome as a function of cell doubling time. J Mol Biol. 1975 May 5;94(1):127–132. doi: 10.1016/0022-2836(75)90410-6. [DOI] [PubMed] [Google Scholar]
- Choung K. K., Estiva E., Bremer H. Genetic and physiological characterization of a spontaneous mutant of Escherichia coli B/r with aberrant control of deoxyribonucleic acid replication. J Bacteriol. 1981 Mar;145(3):1239–1248. doi: 10.1128/jb.145.3.1239-1248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward G., Bremer H. Determination of deoxyribonucleic acid replication time in exponentially growing Escherichia coli B/r. J Bacteriol. 1977 Jun;130(3):1206–1213. doi: 10.1128/jb.130.3.1206-1213.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Dalbow D. G., Bremer H. Metabolic regulation of beta-galactosidase synthesis in Escherichia coli. A test for constitutive ribosome synthesis. Biochem J. 1975 Jul;150(1):1–8. doi: 10.1042/bj1500001b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968 Sep 7;219(5158):1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- Fantes P. A., Grant W. D., Pritchard R. H., Sudbery P. E., Wheals A. E. The regulation of cell size and the control of mitosis. J Theor Biol. 1975 Mar;50(1):213–244. doi: 10.1016/0022-5193(75)90034-x. [DOI] [PubMed] [Google Scholar]
- Geiger L. E., Morris D. R. Polyamine deficiency reduces the rate of DNA replication fork movement in Escherichia coli. Nature. 1978 Apr 20;272(5655):730–732. doi: 10.1038/272730a0. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., Rasmussen K. V. Regulation of the dnaA product in Escherichia coli. Mol Gen Genet. 1977 Oct 20;155(2):219–225. doi: 10.1007/BF00393163. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. II. Analysis of the control mechanism. J Mol Biol. 1974 Mar 25;84(1):21–36. doi: 10.1016/0022-2836(74)90210-1. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. DNA synthesis during the division cycle of three substrains of Escherichia coli B/r. J Mol Biol. 1976 Apr 15;102(3):477–486. doi: 10.1016/0022-2836(76)90329-6. [DOI] [PubMed] [Google Scholar]
- Helmstetter C., Cooper S., Pierucci O., Revelas E. On the bacterial life sequence. Cold Spring Harb Symp Quant Biol. 1968;33:809–822. doi: 10.1101/sqb.1968.033.01.093. [DOI] [PubMed] [Google Scholar]
- Koppes L. H., Woldringh C. L., Nanninga N. Size variations and correlation of different cell cycle events in slow-growing Escherichia coli. J Bacteriol. 1978 May;134(2):423–433. doi: 10.1128/jb.134.2.423-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C. N., Kubitschek H. E. Variation in periodic replication of the chromosome in Escherichia coli B/rTT. J Mol Biol. 1978 Jun 5;121(4):461–471. doi: 10.1016/0022-2836(78)90394-7. [DOI] [PubMed] [Google Scholar]
- Pato M. L. Alterations of the rate of movement of deoxyribonucleic acid replication forks. J Bacteriol. 1975 Jul;123(1):272–277. doi: 10.1128/jb.123.1.272-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci O., Helmstetter C. E. Chromosome replication, protein synthesis and cell division in Escherichia coli. Fed Proc. 1969 Nov-Dec;28(6):1755–1760. [PubMed] [Google Scholar]
- Pritchard R. H., Zaritsky A. Effect of thymine concentration on the replication velocity of DNA in a thymineless mutant of Escherichia coli. Nature. 1970 Apr 11;226(5241):126–131. doi: 10.1038/226126a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Yanofsky C. Metabolic regulation of the tryptophan operon of Escherichia coli: repressor-independent regulation of transcription initiation frequency. J Mol Biol. 1972 Aug 14;69(1):103–118. doi: 10.1016/0022-2836(72)90026-5. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Replication time of the chromosome in thymineless mutants of Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):65–74. doi: 10.1016/0022-2836(71)90447-5. [DOI] [PubMed] [Google Scholar]


