Abstract
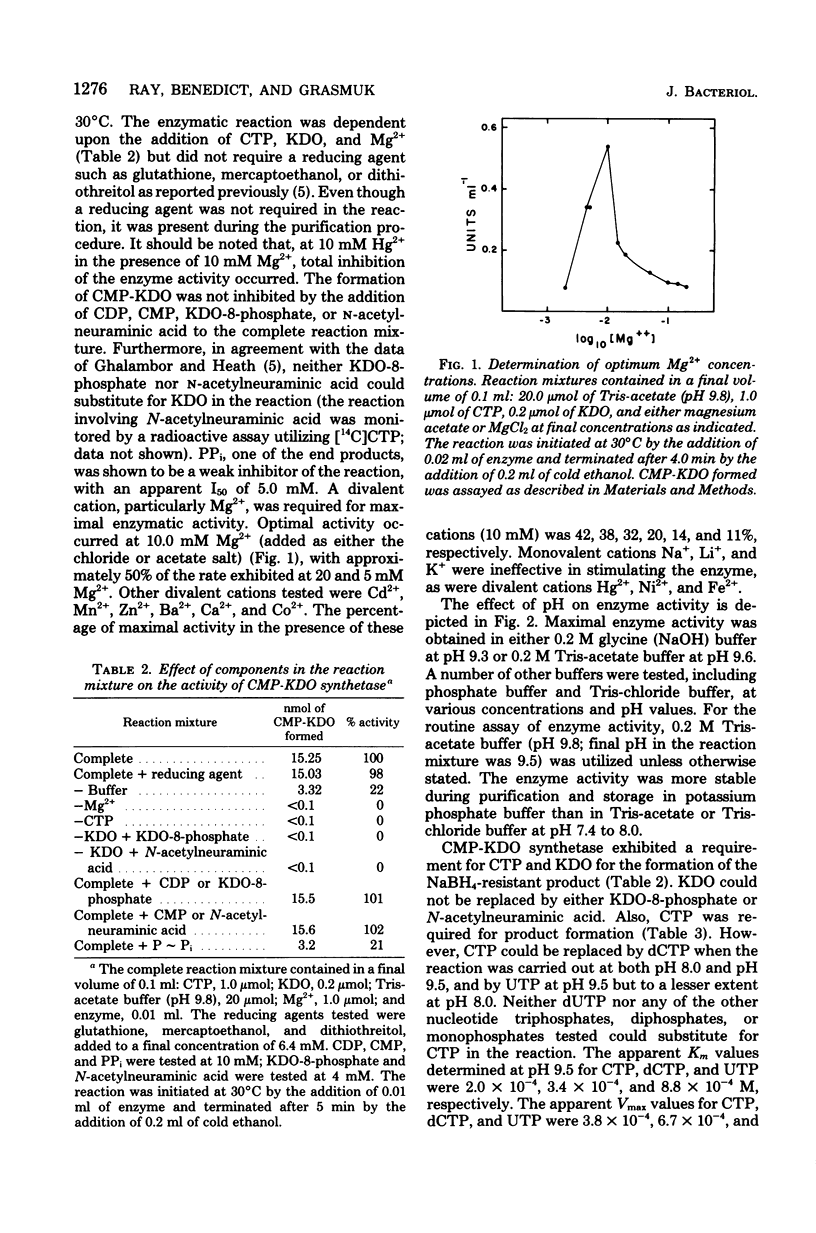
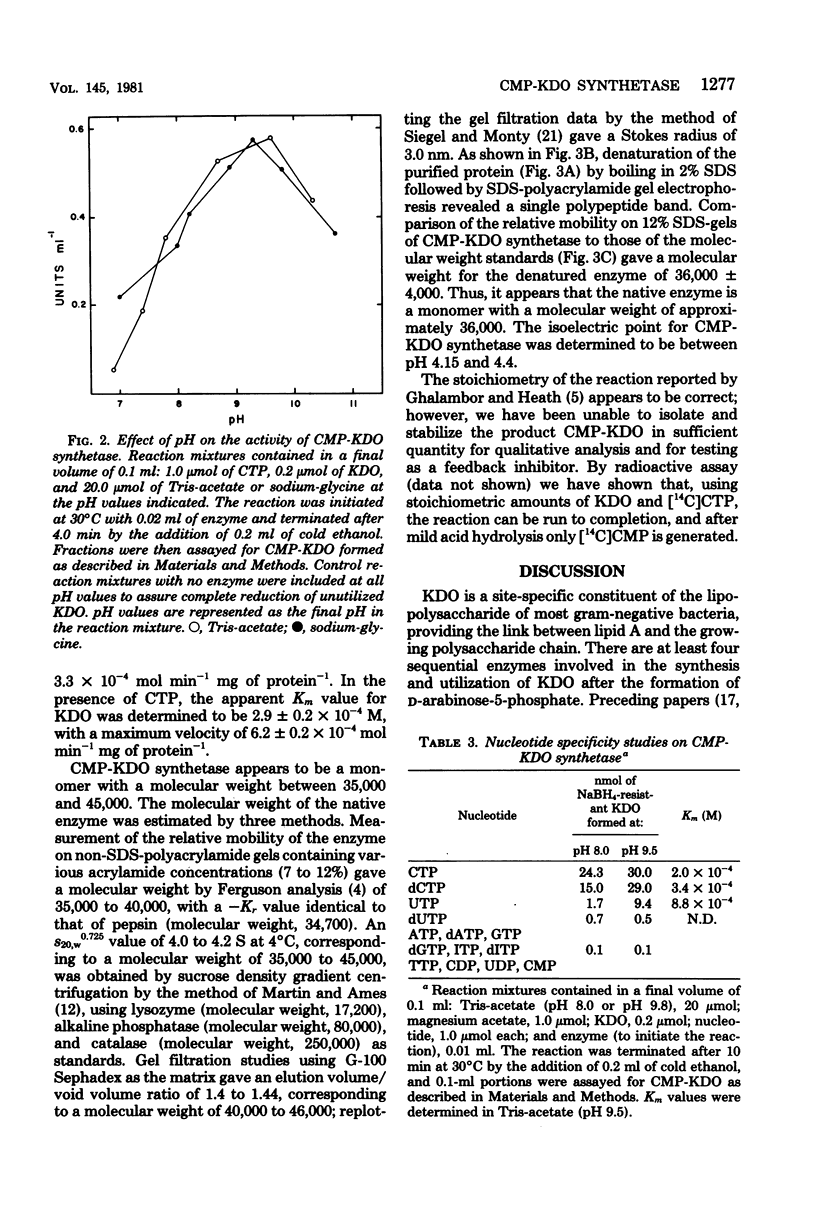
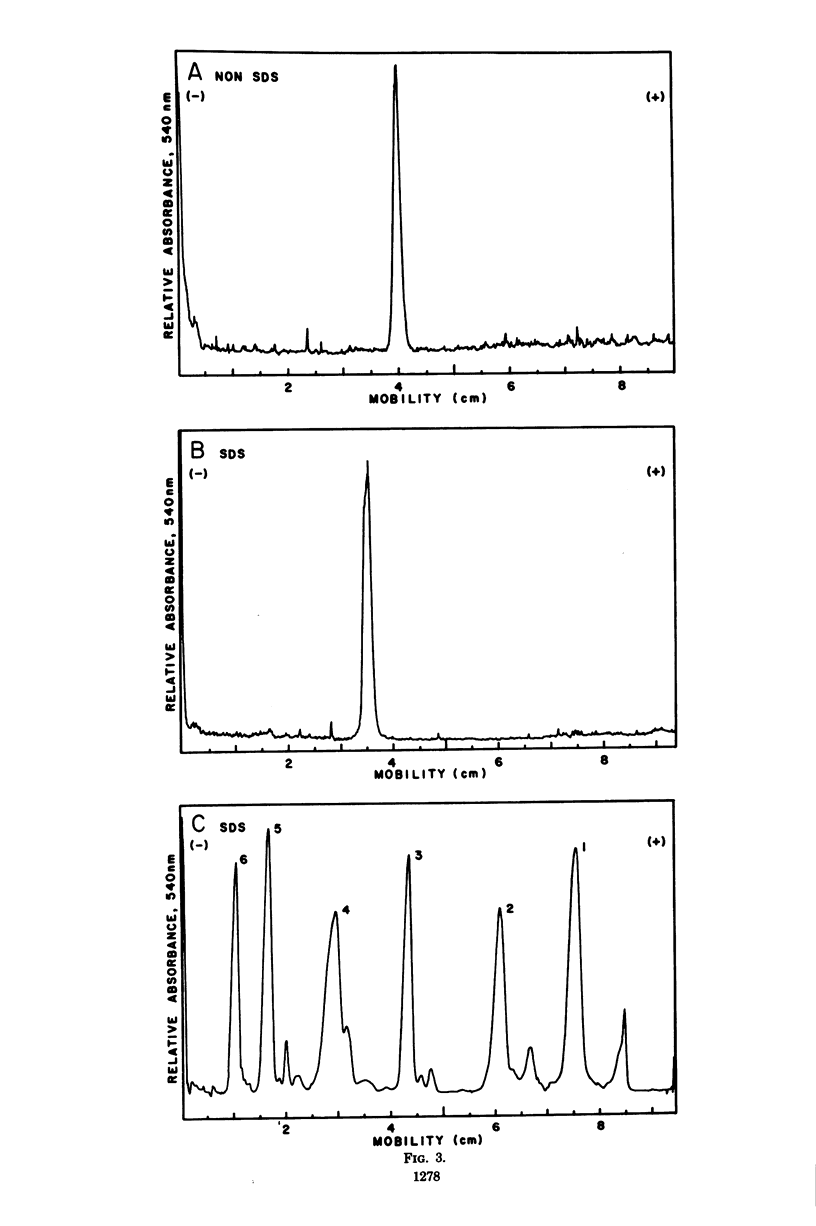
Cytidine 5'-triphosphate:cytidine 5'-monophosphate-3-deoxy-D-manno-octulosonate cytidylyltransferase (CMP-KDO synthetase) was purified 2,300-fold from frozen Escherichia coli B cells. The enzyme catalyzed the formation of CMP-KDO, a very labile product, from CTP and KDO. No other sugar tested could replace KDO as an alternate substrate. Uridine 5'-triphosphate at pH 9.5 and deoxycytidine 5'-triphosphate at pH 8.0 and 9.5 could be used as alternate substrates in place of CTP. CMP-KDO synthetase required Mg2+ at a concentration of 10.0 mM for optimal activity. The pH optimum was determined to be between 9.6 and 9.3 in tris(hydroxymethyl)aminomethane-acetate or sodium-glycine buffer. This enzyme had an isoelectric point between pH 4.15 and 4.4 and appeared to be a single polypeptide chain with a molecular weight of 36,000 to 40,000. The apparent Km values for CTP and KDO in the presence of 10.0 mM Mg2+ were determined to be 2.0 X 10(-4) and 2.9 X 10(-4) M, respectively, at pH 9.5. Uridine 5'-triphosphate and deoxycytidine 5'-triphosphate had apparent Km values of 8.8 X 10(-4) and 3.4 X 10(-4) M. respectively, at pH 9.5.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Ghalambor M. A., Heath E. C. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. IV. Purification and properties of cytidine monophosphate 3-deoxy-d-manno-octulosonate synthetase. J Biol Chem. 1966 Jul 10;241(13):3216–3221. [PubMed] [Google Scholar]
- Hershberger C., Davis M., Binkley S. B. Chemistry and metabolism of 3-deoxy-D-mannoctulosonic acid. II. Practical synthesis and stability. J Biol Chem. 1968 Apr 10;243(7):1585–1588. [PubMed] [Google Scholar]
- LEVIN D. H., RACKER E. Condensation of arabinose 5-phosphate and phosphorylenol pyruvate by 2-keto-3-deoxy-8-phosphooctonic acid synthetase. J Biol Chem. 1959 Oct;234:2532–2539. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim R., Cohen S. S. D-phosphoarabinoisomerase and D-ribulokinase in Escherichia coli. J Biol Chem. 1966 Oct 10;241(19):4304–4315. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Munson R. S., Jr, Rasmussen N. S., Osborn M. J. Biosynthesis of lipid A. Enzymatic incorporation of 3-deoxy-D-mannooctulosonate into a precursor of lipid A in Salmonella typhimurium. J Biol Chem. 1978 Mar 10;253(5):1503–1511. [PubMed] [Google Scholar]
- Osborn M. J., Rick P. D., Rasmussen N. S. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Translocation and integration of an incomplete mutant lipid A into the outer membrane. J Biol Chem. 1980 May 10;255(9):4246–4251. [PubMed] [Google Scholar]
- Ray P. H., Benedict C. D. Purification and characterization of specific 3-deoxy-D-manno-octulosonate 8-phosphate phosphatase from Escherichia coli B. J Bacteriol. 1980 Apr;142(1):60–68. doi: 10.1128/jb.142.1.60-68.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. H. Purification and characterization of 3-deoxy-D-manno-octulosonate 8-phosphate synthetase from Escherichia coli. J Bacteriol. 1980 Feb;141(2):635–644. doi: 10.1128/jb.141.2.635-644.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick P. D., Fung L. W., Ho C., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Purification and characterization of a lipid A precursor produced by a mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4904–4912. [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Isolation of a mutant of Salmonella typhimurium dependent on D-arabinose-5-phosphate for growth and synthesis of 3-deoxy-D-mannoctulosonate (ketodeoxyoctonate). Proc Natl Acad Sci U S A. 1972 Dec;69(12):3756–3760. doi: 10.1073/pnas.69.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Walenga R. W., Osborn M. J. Biosynthesis of lipid A. Formation of acyl-deficient lipopolysaccharides in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1980 May 10;255(9):4257–4263. [PubMed] [Google Scholar]
- Walenga R. W., Osborn M. J. Biosynthesis of lipid A. In vivo formation of an intermediate containing 3-deoxy-D-mannoctulosonate in a mutant of Salmonella typhimurium. J Biol Chem. 1980 May 10;255(9):4252–4256. [PubMed] [Google Scholar]


